Position statement
Guidelines for detection and management of hyperbilirubinemia in term and late preterm newborns (≥35 weeks gestational age)
Posted: Mar 18, 2025
Principal author(s)
Eugene Ng, Gabriel Altit, Chloe Joynt, Nicole Radziminski, Michael Narvey, Fetus and Newborn Committee
Abstract
Hyperbilirubinemia is a common condition and is usually benign in term and late preterm newborns. Developing a standardized approach for all newborns to prevent, identify, and manage those with clinically significant hyperbilirubinemia will minimize the potential risks of long-term neurological sequelae from acute bilirubin encephalopathy and kernicterus. This statement provides recommendations on universal screening, risk factors assessment, and approaches to hyperbilirubinemia treatment, investigations, and monitoring.
Keywords: Bilirubin; Exchange transfusion; Hemolysis; Hyperbilirubinemia; Jaundice; Neonates; Phototherapy
BACKGROUND AND EPIDEMIOLOGY
Approximately 10% of term and preterm newborns have a clinically significant elevation of total serum bilirubin (TSB) levels requiring close surveillance or treatment[1][2]. Standardization of screening and management strategies has significantly decreased the incidence of severe neonatal hyperbilirubinemia, defined as a peak TSB >425 µmol/L or need for a blood exchange transfusion (BET). Following the publication of the 2007 Canadian Paediatric Society (CPS) position statement[3], the Canadian Paediatric Surveillance Program (CPSP) reported a more than threefold decline in the incidence of severe hyperbilirubinemia[4][5]. However, kernicterus continues to be reported in North America and Europe[6]-[11], and hyperbilirubinemia requiring treatment is still the predominant reason for hospital readmissions of newborns in North America[4].
This statement replaces the 2007 CPS document on hyperbilirubinemia[3]. The American Academy of Pediatrics (AAP) published revised guidance in 2022 with new recommendations for the prevention, assessment, and management of neonatal hyperbilirubinemia[12]. Their recommendations were based on current best evidence and, when that was lacking, on consensus and expert opinion. Several elements from the AAP guideline have been incorporated into the new CPS statement, specifically:
- A universal screening strategy to identify neonates at risk for developing severe hyperbilirubinemia,
- An algorithm to assess and monitor neonates for hyperbilirubinemia,
- Treatment, investigation, and monitoring protocols for neonates with hyperbilirubinemia, and
- Follow-up for neonates treated for hyperbilirubinemia following hospital discharge.
SCREENING FOR HYPERBILIRUBINEMIA
Screening for isoimmune hemolytic disease
Neonatal isoimmune hemolytic disease from red cell incompatibilities is a leading cause of severe hyperbilirubinemia in newborns. During routine prenatal care, universal screening including ABO blood group, RhD type, and antibody screen should be performed in early pregnancy[13]. Antibody screening identifies alloantibodies to red cell antigens. If results at time of delivery are unavailable or unknown, or if the maternal antibody screen is positive, early neonatal testing should include blood type (ABO and RhD), red blood cell antigen testing for the cognate antibody (if maternal antibody screen is positive), a direct antiglobulin test (DAT), and total serum bilirubin (TSB). Cord sample is preferable although peripheral blood samples can be used.
There is insufficient evidence to support a universal DAT screening strategy for all newborns, or for newborns of group O-positive mothers, to identify infants at risk for significant hyperbilirubinemia caused by immune-mediated hemolysis[14]-[17]. The DAT detects the presence of antibodies on the red blood cell surface, but a positive test does not imply hemolysis, nor does it measure the strength of hemolysis[18]. A false-positive DAT can be seen in infants whose mothers received Rh immune globulin (RhIG), and can be falsely negative after profound hemolysis or antibody clearance[19]. Clinicians should be aware of these limitations when interpreting the DAT. Hemolysis is best confirmed using the DAT in conjunction with other investigations, such as hemoglobin, blood smear, reticulocyte count, and bilirubin, when readily available. These tests should be performed in newborns suspected to have hemolysis due to an early, rapidly rising TSB or severe hyperbilirubinemia[20][21].
RECOMMENDATIONS:
- Test for maternal blood group (ABO and Rh) and red cell antibody screen as part of routine prenatal care. When these results are unavailable or unknown, make every effort to perform these tests before delivery.
- If the maternal antibody screen is positive or unknown at time of delivery, newborn testing should include a total serum bilirubin (TSB), hemoglobin, reticulocyte count, blood smear, direct antiglobulin test (DAT), and blood group, preferably obtained from cord blood. Monitor these neonates closely for the development of early (<24 hours postnatal age) hyperbilirubinemia.
Universal screening strategy to predict hyperbilirubinemia
The goal of universal screening is to identify newborn infants at risk for developing hyperbilirubinemia. The AAP[6] and CPS[3] have previously recommended screening all healthy newborns with a timed TSB between 18 to 72 hours of age and plotting the result on a predictive nomogram to facilitate post-discharge follow-up[22]. A new approach is proposed using the delta-TSB (△TSB): the difference between the timed TSB concentration and the phototherapy threshold at age of sampling to determine the most appropriate care plan[12] (Figure 1). The △TSB better predicts development of clinically significant hyperbilirubinemia requiring treatment than the original predictive nomogram[23].
Risk factor assessment
Two categories of jaundice risk factors have been described, with some overlap between them. Hyperbilirubinemia risk factors (Table 1a) increase baseline risk of a neonate’s developing this condition[6][10][24]-[27], while neurotoxicity risk factors (Table 1b) increase the toxic effects of bilirubin on the newborn brain. In isolation each factor is of limited predictive value, but these risks are additive[24]. While knowing that hyperbilirubinemia risk factors are present during the care of a newborn is important, only the neurotoxicity risk factors are incorporated into the phototherapy and exchange transfusion thresholds.
Determining neurotoxicity risk in a newborn requires clinical judgement. Some neurotoxicity risk factors are based on the hypothesis that the blood-brain barrier is compromised in newborns with a serious illness (e.g., sepsis[28][29]), which supports the use of lower TSB thresholds to initiate phototherapy and mitigate neurotoxicity risk. Similarly, hypoalbuminemia reduces bilirubin binding and may potentiate the neurotoxic effect of hyperbilirubinemia[30]. While there is insufficient evidence to recommend routine measurement of serum albumin in all newborns, this measure may be helpful for guiding management in seriously ill newborns or in those whose hyperbilirubinemia is severe (i.e., within reach of the pre-exchange transfusion threshold)[12].
|
Table 1a. Risk factors for significant hyperbilirubinemia (hyperbilirubinemia risk factors) |
|
|
DAT Direct antiglobulin test; G6PD Glucose-6-phosphate dehydrogenase |
|
Table 1b. Risk factors that increase the neurotoxic effects of bilirubin (neurotoxicity risk factors) |
|
|
*Hemolysis may be suspected based on a rapid rate of increase in TSB ≥5 µmol/L/h (within 24 hours post-birth) or ≥3.5 µmol/L/h (beyond 24 hours post-birth). Information drawn from reference[12] |
Timed TSB measurement to determine the ΔTSB
The new phototherapy thresholds based on gestational age (GA) and the presence of bilirubin neurotoxicity risk factors are described below (Figures 2, 3). Calculating the △TSB in newborns between 12 and 120 hours of age indicates how close the measured TSB value is to the threshold for phototherapy treatment: The smaller the △TSB, the closer an infant is to requiring phototherapy treatment. Transcutaneous bilirubin (TcB) values can also be used (see the Bilirubin measurement section for the correlation between TcB and TSB).
|
△TSB = Age(in hours)-specific phototherapy threshold - Measured TSB or TcB For example, a 24-hour-old infant born at 37 weeks gestation has a TSB screen of 150 µmol/L. The phototherapy threshold at 24 hours for this infant (per Figure 2) is 200 µmol/L. Therefore, the △TSB is 200 µmol/L - 150 µmol/L = 50 µmol/L. |
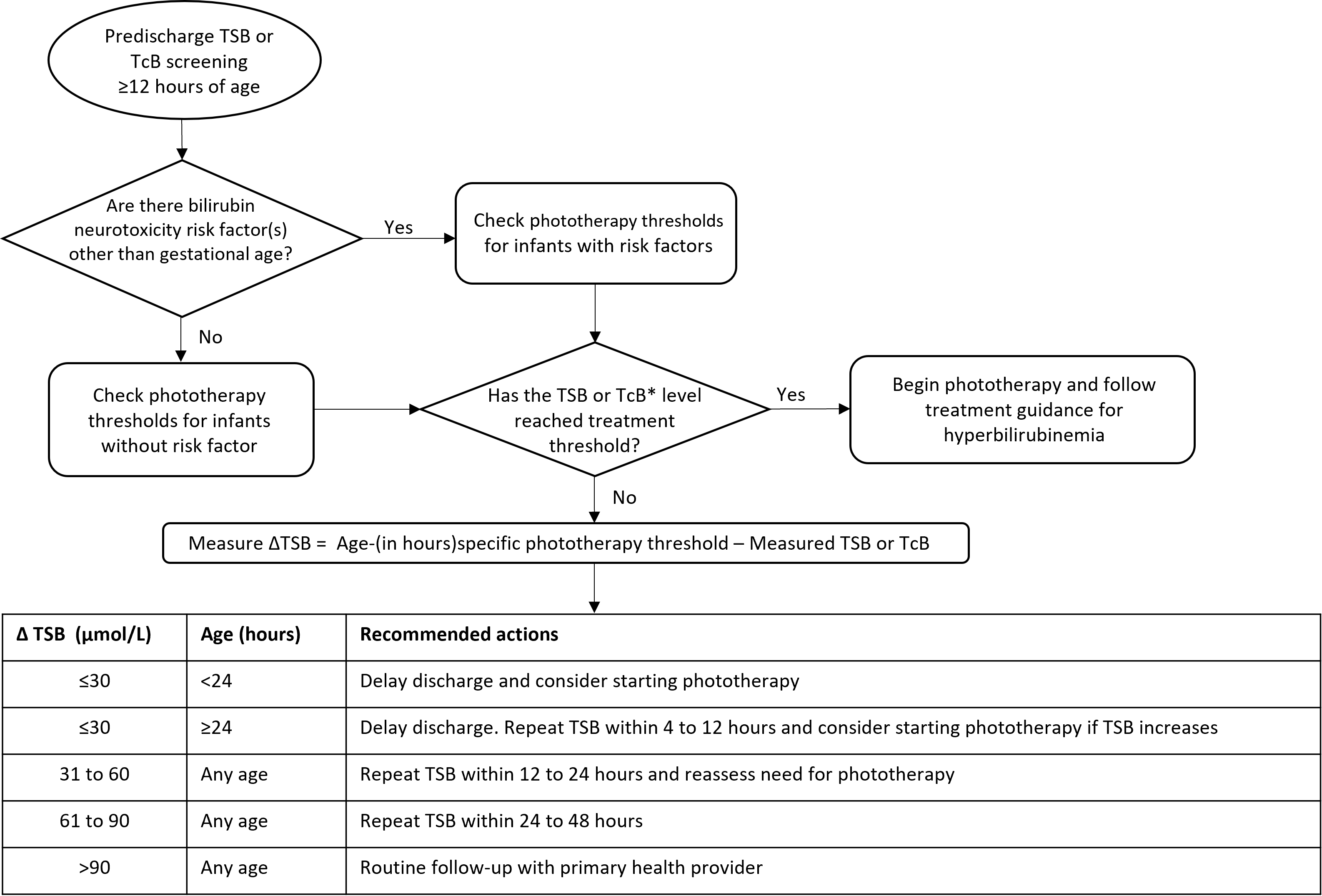
The △TSB should be used to guide discharge planning: first to determine whether discharge should be delayed pending further TSB measurement(s) or phototherapy treatment, then if an infant is eligible for discharge, to time follow-up. Measuring △TSB for screening purposes applies only to newborns who are at least 12 hours of age who have not received phototherapy. For home births or discharge before 12 hours of age, arrangements should be made for a bilirubin screen to be performed within the recommended period. See Figure 1 for an algorithm.
Remember that the key action in this algorithm is to delay discharge or initiate phototherapy (or both) when an infant’s △TSB is ≤30 µmol/L [The first rule of 30].
RECOMMENDATIONS:
- Measure a pre-discharge total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) in all newborns at a minimum of 12 hours post-birth. When there is no clinical concern for early jaundice, the TSB can be obtained at the same time as the newborn metabolic screen to minimize the number of painful procedures.
- If the screening TSB or TcB concentration is below treatment threshold, calculate the △TSB to determine appropriate management before discharge is contemplated (per Figure 1).
Figure 1. Algorithm for universal hyperbilirubinemia screening at 12 to 120 hours of age.
*Confirm with TSB if TcB is within 50 µmol/L of phototherapy threshold
TcB, transcutaneous bilirubin; TSB, total serum bilirubin
Anticipatory guidance for families
Pre-discharge checklists should include written and verbal information about jaundice, along with screening results (i.e., the timed TSB or TcB level[s], the time[s] they were taken, the △TSB, and recommended next steps) and clearly communicated to caregivers, preferably in their first language, such that post-discharge follow-up with their primary health provider(s) can be arranged[31][32].
RECOMMENDATION:
- Provide families with first language concordant verbal and written information about neonatal jaundice, including screening results and interpretation to facilitate post-discharge follow-up.
Breastfeeding support
Although breastfed infants are at a higher risk for developing clinically significant hyperbilirubinemia than formula-fed infants[33]-[35], the risks of complications from hyperbilirubinemia are small when weighed against the myriad benefits of breastfeeding and human milk feeding[36][37]. Protective measures against hyperbilirubinemia include early skin-to-skin contact and breastfeeding, and evaluating feeding effectiveness by monitoring the infant’s weight and urine and stool outputs[38]-[41]. Providing breastfeeding education and support in-hospital and in-community is part of essential care for families choosing to breastfeed exclusively, and they should be discharged with information on local resources and supportive services[42].
RECOMMENDATION:
-
To help prevent jaundice associated with suboptimal oral intake and excessive weight loss, breastfeeding support should be accessible to all newborns and parents until feeding is well established.
CLINICAL ASSESSMENT OF HYPERBILIRUBINEMIA
Clinical surveillance for hyperbilirubinemia in healthy newborns
All newborns should have a physical assessment within the first 24 hours post-birth by a health care provider (HCP)[3][6]. In addition to universal screening with a timed TSB or TcB, clinical surveillance to identify neonates at risk for significant hyperbilirubinemia should be part of all newborn assessments. Identification points include hyperbilirubinemia and bilirubin neurotoxicity risk factors, a review of feeding, stooling, and voiding adequacy, weight assessments, and visual assessments for jaundice (i.e., icterus on skin and sclera).
Peak TSB concentration generally occurs between 72 and 120 hours of age, when most healthy newborns are already home from hospital[43]. A process must be in place to ensure timely post-discharge follow-up of newborns at intervals based on the △TSB, such that jaundiced infants can be assessed and treated promptly. While the absence of jaundice on visual assessment in the first 48 hours post-birth is highly predictive against the development of significant hyperbilirubinemia[44], it is also true that visual inspection for the extent of icterus, particularly in infants with a darker skin tone, is unreliable[36]. The appearance of clinical jaundice should prompt the clinician to perform a TSB or TcB.
RECOMMENDATIONS:
- Every newborn should have a thorough clinical assessment within 24 hours of birth, including assessment for hyperbilirubinemia and bilirubin neurotoxicity risk factors (Tables 1a, 1b) and clinical assessment for jaundice.
- A process should be in place for post-discharge follow-up of newborns at risk for hyperbilirubinemia, including resources to support timely TSB or TcB measurement(s) and mechanisms to provide prompt treatment of hyperbilirubinemia when required.
- Any newborn with suspected significant hyperbilirubinemia on assessment, at any age, should have their TSB or TcB measured without delay.
Bilirubin measurement
TSB can be measured by venous or capillary samples, with acceptable correlation between the two methods[45][46]. Capillary sampling is the more common and studied technique for the detection of hyperbilirubinemia[47]. TcB measurement can provide a reasonable estimate of TSB levels when the TSB is <250 µmol/L[48]-[51]. Expert opinion recommends a confirmatory TSB when the TcB falls within 50 µmol/L of the phototherapy threshold.
There are many commercially available transcutaneous bilirubinometers, and comparative studies have found inter-device variability as high as 65 µmol/L, particularly within clinically relevant ranges[52]. Each newly acquired device should be lab tested in-house for serum correlation in multiple samples before it is deployed, and calibrated periodically as per manufacturer recommendations[48]. Should bias be discovered, the corrective factor must be applied to each TcB measurement before plotting it on phototherapy threshold graphs to avoid under- or overestimating TSB values[53][54].
RECOMMENDATION:
- When TcB testing is used to monitor infants with hyperbilirubinemia, obtain confirmatory testing with TSB if the TcB level is within 50 µmol/L of the hour-specific phototherapy threshold OR if the TcB is above 250 µmol/L.
MANAGING HYPERBILIRUBINEMIA
Phototherapy
The efficacy of phototherapy depends on light source intensity, the distance between light source and skin, the surface area being exposed, and duration of treatment. Intensive phototherapy is defined by using narrow spectrum lights at a wavelength of around 460 to 490 nm with an irradiance of at least 30 µW/cm2/nm[55]. The current recommended approach is to provide intensive phototherapy for any infant who reaches threshold based on hour-specific TSB values (Figures 2, 3)[12]. Use of additional light sources, including a fiberoptic blanket or pad, may increase the body surface area exposed without necessarily increasing delivered irradiance. Because irradiance decay occurs in all types of phototherapy lamps with ongoing use, devices should be checked regularly by measuring the spectral irradiance using a radiometer[56].
Phototherapy thresholds
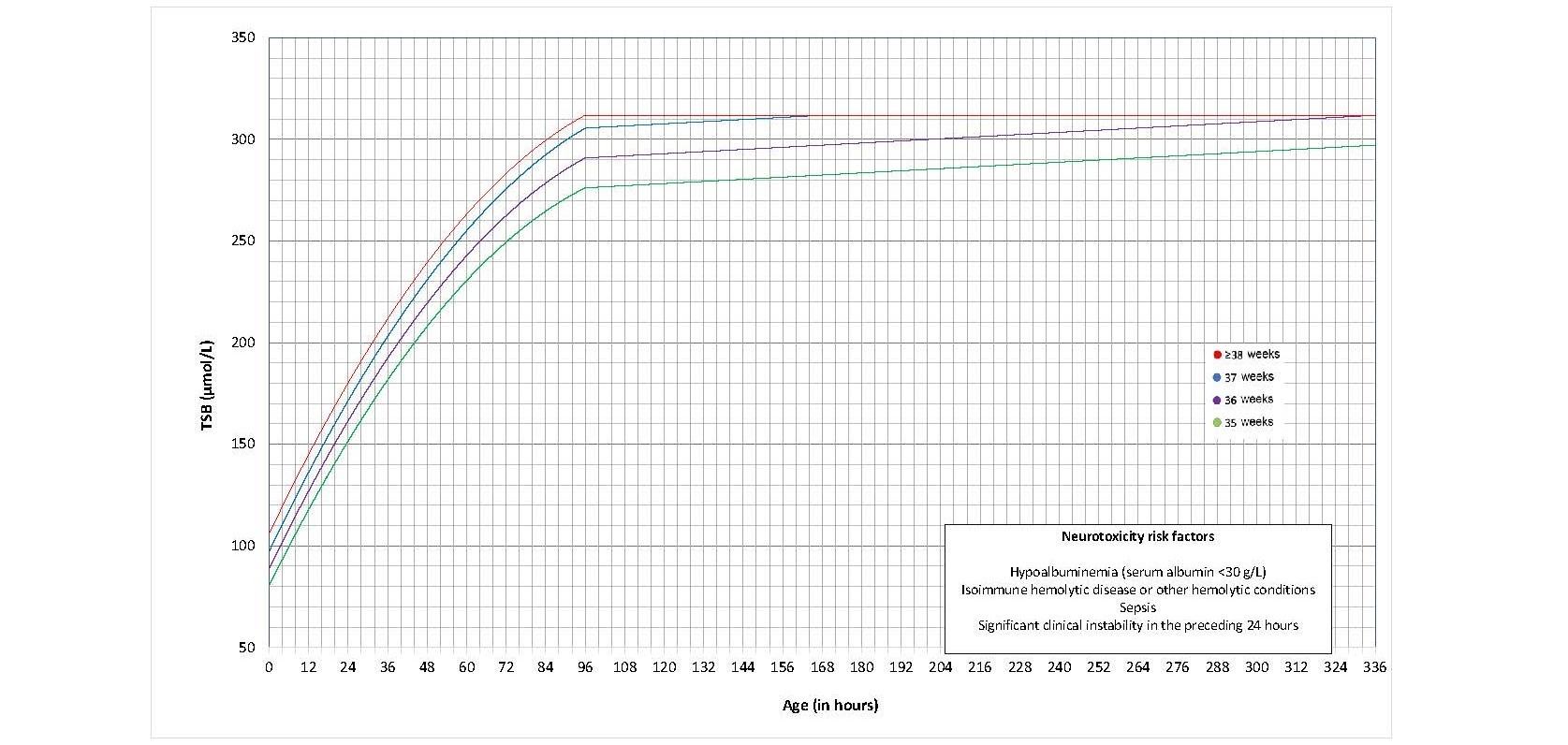
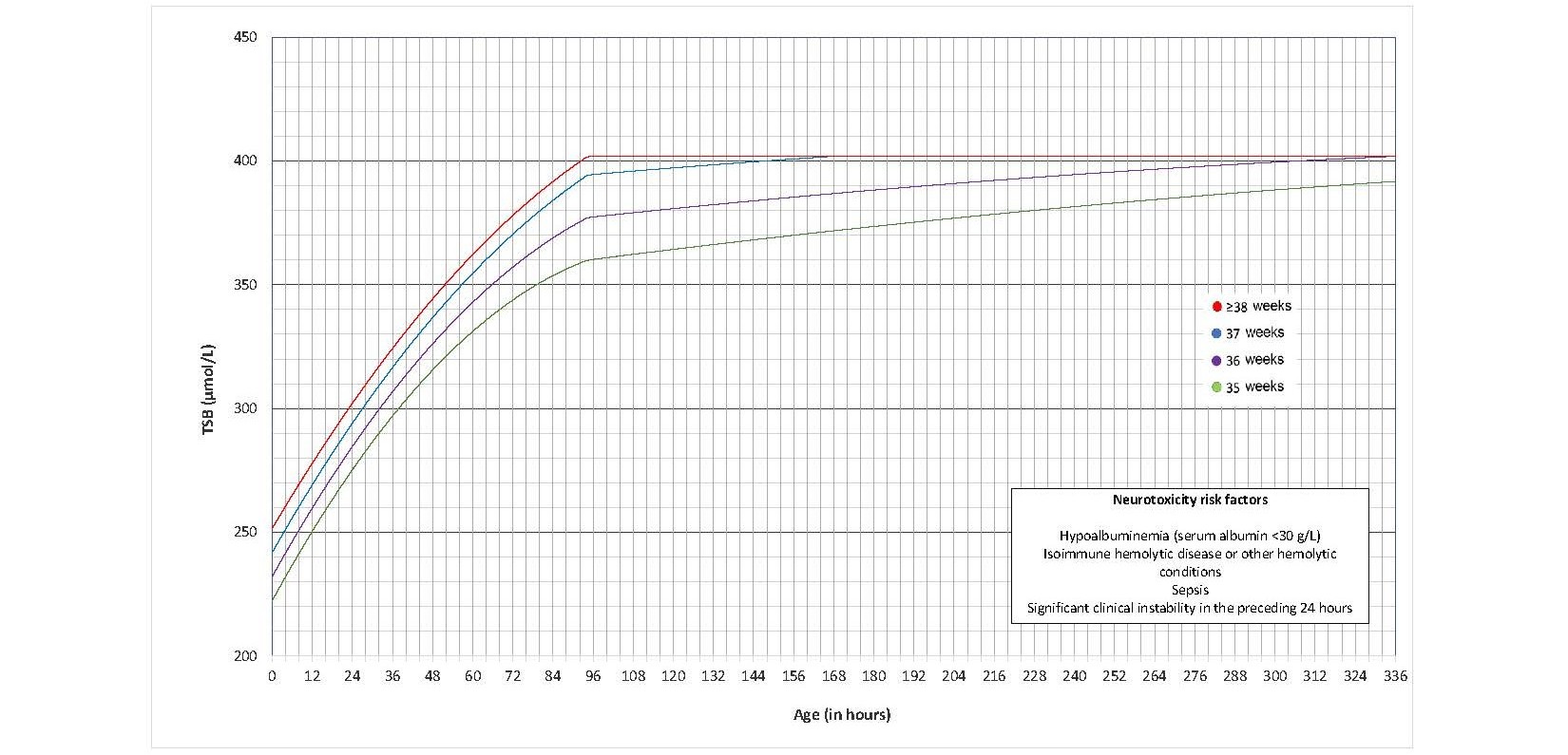
The AAP’s 2022 guideline introduced new thresholds for initiating phototherapy[12] that were narrowly higher than those recommended previously[3][6]. They balanced findings that bilirubin neurotoxicity and kernicterus occur at levels well above previously recommended thresholds against potential adverse effects from phototherapy[57]. There are separate graphs for infants with and without neurotoxicity risk factors, and phototherapy thresholds were developed for each GA at birth (defined in Figures 2, 3, 6, and 7 as completed weeks) from 35 to 40 weeks.
Figure 2. Phototherapy thresholds for infants with no neurotoxicity risk factor(s) (data provided by the AAP and used with permission)
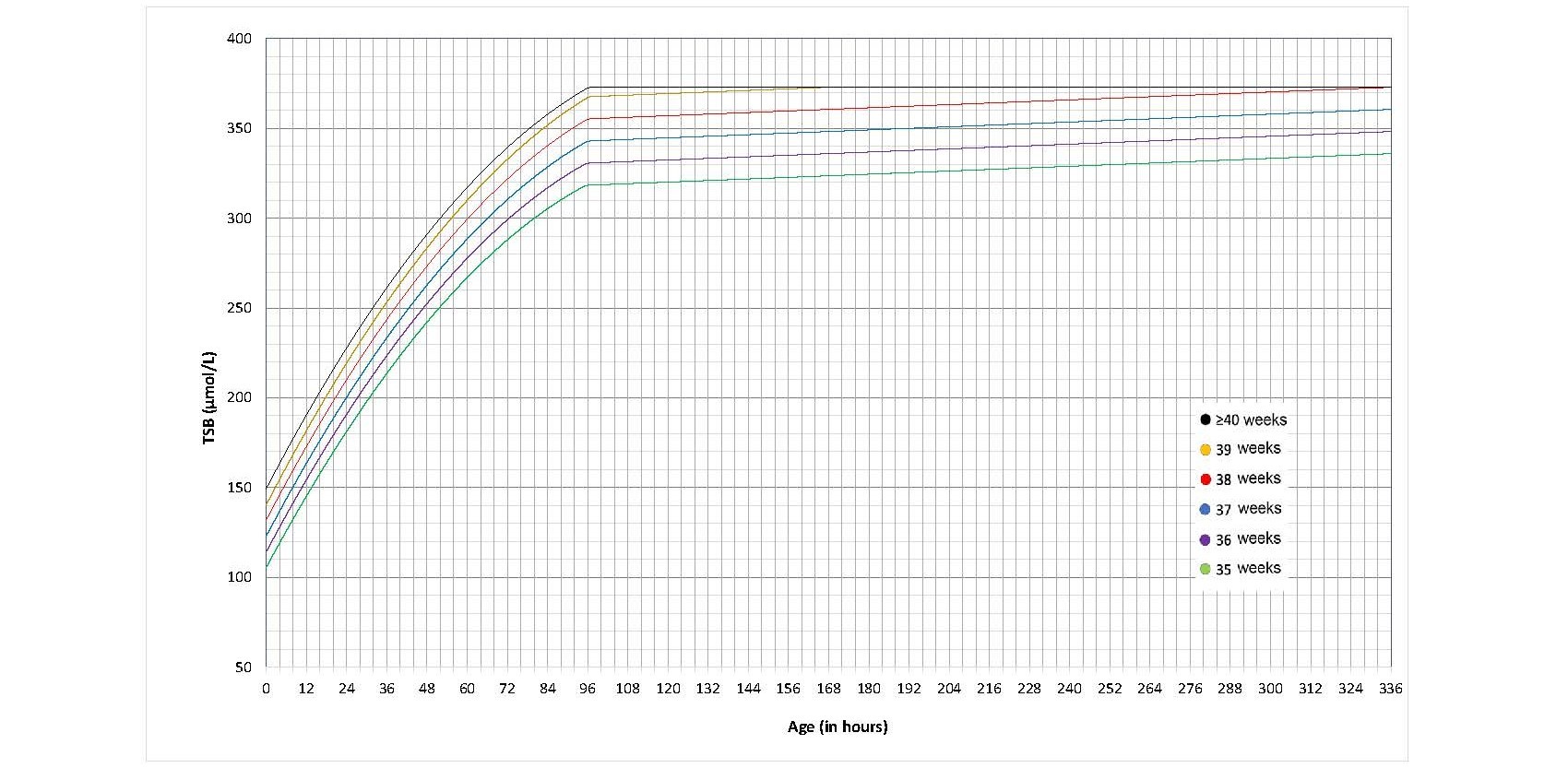
Figure 3. Phototherapy thresholds for infants with neurotoxicity risk factor(s) (data provided by the AAP and used with permission)
To clarify how far these new thresholds deviated from previous guidelines, see Figures S1a-c in the Technical Annex[58].
While changes to previous phototherapy thresholds are relatively small, phototherapy can be initiated at the clinician’s discretion for infants with neurotoxicity risk factor(s) when TSB is ≤30 µmol/L of the current phototherapy threshold [The second rule of 30].
Supplemental fluids
Newborns with hyperbilirubinemia may also experience dehydration related to feeding challenges[9][59]. Evidence suggests that supplementation with intravenous (IV) fluids by 10% to 25% above daily maintenance can reduce TSB over 4 to 8 hours. Oral and IV fluids supplementation are equally effective toward reducing TSB levels[60]. Increasing enteral intake, including the use of gavage feeding, has the added benefits of increasing gut transit and reducing enterohepatic circulation of bilirubin[61][62]. IV fluids supplementation should be reserved for infants with severe dehydration or with higher risk for requiring a BET (refer to the pre-exchange transfusion protocol, Figure 5).
Adverse effects of phototherapy
Several recent studies have suggested a measure of risk for adverse outcomes related to phototherapy, such as asthma, allergies, hematopoietic malignancies, and epilepsy[57]. However, confounders and study design have limited all these associations (see the Technical Annex for details). Because phototherapy is a proven treatment to reduce risk for adverse neurodevelopmental outcomes from severe hyperbilirubinemia, clinicians need to interpret these and similar studies with caution.
Family-centred care during phototherapy
Phototherapy can interfere with breastfeeding and skin-to-skin care and may generate parental anxiety related to the hospitalization of an otherwise healthy newborn[63][64]. Encourage family involvement with treatment[64] by briefly interrupting phototherapy for feeding at the breast or skin-to-skin care (for 20 to 30 minutes, 8 to 10 times per day can be considered in the mild hyperbilirubinemia setting[65]). Using a fiberoptic blanket or pad during phototherapy helps allow breastfeeding and skin-to-skin contact to continue[66].
RECOMMENDATIONS:
- Provide intensive phototherapy to all infants who reach treatment threshold (Figures 2, 3) based on GA, the presence of neurotoxicity risk factor(s) (Table 1b), and the infant’s age in hours.
- For infants with neurotoxicity risk factors, consider initiating phototherapy when TSB is ≤30 μmol/L of the phototherapy threshold.
- Depending on presentation and degree of hyperbilirubinemia, feeding at the breast and skin-to-skin care can often continue during phototherapy with the help of a fiberoptic blanket or pad.
Monitoring infants during phototherapy
Serial measurements are the only way to monitor the TSB trajectory in infants undergoing phototherapy for hyperbilirubinemia. TSB trajectory can be calculated by its rate of rise, defined as the difference between two sequential TSB values at least 3 hours apart, divided by the time elapsed between the two samples[23][67]. For example, a newborn with suspected hemolytic disease has a TSB of 120 µmol/L at 4 hours of age. By 8 hours of age, the TSB has risen to 160 µmol/L. The rate of rise was (160 µmol/L-120 µmol/L)/4 hours = 10 µmol/L/h.
Calculating the TSB trajectory allows clinicians to decide whether to discontinue phototherapy or escalate treatment. The frequency of TSB measurements during phototherapy is determined based on infant’s age, the presence of risk factors for hyperbilirubinemia and neurotoxicity, and TSB trajectory. With the exception of infants whose TSB is within range of the pre-exchange transfusion threshold, repeating a TSB within 12 to 24 hours is recommended.
In neonates who do not have hemolytic risk factor(s), the TSB would be expected to fall or plateau after initiating phototherapy. However, when TSB level continues to rise despite phototherapy, or the TSB rate of rise is ≥5 µmol/L/h (in the first 24 hours of age) or ≥3.5 µmol/L/h (beyond 24 hours of age), hemolysis can be suspected[27]. Further investigations may include some or all of the following: CBC, reticulocyte count, blood smear examination for red cell membrane defects, DAT or other indices of hemolysis, and G6PD and pyruvate kinase essays. Depending on clinical presentation, investigations may also include evaluation for sepsis or urinary tract infection (or both), and the identification of rare red cell antibodies by a blood service organization.
Infants with an abnormal rate of rise or suspected hemolysis should be monitored by TSB at least every 4 to 6 hours to assess need for escalation of treatment to the pre-exchange transfusion protocol (Figure 5). Always check to ensure such infants are receiving optimal phototherapy treatment by evaluating the irradiance level, the distance between the light source and the infant’s body, and the skin area exposed, and by minimizing interruptions during phototherapy sessions.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency
G6PD deficiency is an X-linked recessive red cell enzyme defect[68], now recognized as a leading cause of kernicterus from severe neonatal hyperbilirubinemia[4][69][70]. Clinicians should consider this diagnosis in settings of persistent jaundice because most affected infants do not have a significant family history of G6PD deficiency[71]. Consider G6PD screening or assay for all infants with hyperbilirubinemia unresponsive to phototherapy or occurring in the absence of risk factors. In cases of active hemolysis, G6PD activity levels can be overestimated because the circulating red blood cells may still have enough G6PD to sustain oxidative stress, leading to a false-negative result. When G6PD deficiency is suspected but not confirmed (G6PD activity normal or near normal), testing should be repeated at 3 months of age.
RECOMMENDATIONS:
- For hospitalized infants undergoing phototherapy, TSB should be measured within 12 to 24 hours of initiating treatment. The frequency of TSB monitoring thereafter is determined by age, the presence of risk factor(s), and TSB trajectory.
- If an infant’s TSB continues to rise or is not responding to intensive phototherapy, additional tests could include CBC, reticulocyte count, blood smear, direct antiglobulin test, G6PD or pyruvate kinase essay, and sepsis screening if clinically indicated. Measure TSB at least every 4 to 6 hours to assess need to initiate the pre-exchange transfusion protocol.
Figure 4. How infants should be monitored during phototherapy
*Check to ensure adequate irradiance, proximity of light source to the skin, and area of exposure, and that infant is receiving phototherapy with minimal interruptions.
†Pre-exchange transfusion threshold is TSB ≤30 µmol/L of exchange transfusion threshold.
CBC, Complete blood count; DAT, Direct antiglobulin test; GA, gestational age; G6PD, Glucose-6-phosphate dehydrogenase; PK, Pyruvate kinase; TSB, Total serum bilirubin
When to stop phototherapy
When an infant’s TSB decreases from pre-treatment level to below the age-specific treatment threshold, phototherapy can be stopped. Rebound hyperbilirubinemia can occur and is defined as the TSB reaching phototherapy threshold again within 72 to 96 hours of stopping phototherapy.
GA (<38 weeks), age at initiation of phototherapy (<48 hours), and the △TSB are the most robust predictors of rebound hyperbilirubinemia[72]-[74]. In one large cohort study, discontinuation of phototherapy at a △TSB of >30 µmol/L minimized the rate of rebound hyperbilirubinemia to <3% in infants born ≥38 weeks GA and <11% for those born 35 to 37 weeks GA [73].
For infants born ≥38 weeks GA, continuing phototherapy until TSB is >30 µmol/L below the phototherapy threshold helps minimize risk for rebound hyperbilirubinemia. For infants born 35 to 37 weeks GA, using a higher △TSB of >60 µmol/L (or >2 x 30 µmol/L) may increase the protective margin against rebound hyperbilirubinemia [The third rule of 30].
Re-measuring TSB no sooner than 12 to 24 hours after stopping phototherapy will detect rebound hyperbilirubinemia if it occurs[75]. For high-risk infants (e.g., those with a hemolytic disease), follow-up within 6 to 12 hours and every 24 hours thereafter is recommended, until TSB is on a downward trend and △TSB is well above 60 µmol/L[76]-[78]. TcB can be used for follow-up provided that at least 18 hours have elapsed since phototherapy was discontinued[50].
RECOMMENDATIONS:
- Discontinue phototherapy at △TSB >30 µmol/L below treatment threshold for infants born ≥38 weeks GA and at △TSB >60 µmol/L for those born 35 to 37 weeks GA to minimize risk for rebound hyperbilirubinemia.
- Measure TSB after stopping phototherapy to check for rebound hyperbilirubinemia. TcB can be used for follow-up if phototherapy has been discontinued for ≥18 hours.
Prolonged jaundice
Prolonged jaundice is defined as clinically significant jaundice, where TSB levels are within 35 µmol/L of the phototherapy threshold and persist beyond 14 days of age in term or late preterm infants[12][79]. Although prolonged jaundice may be caused by conditions of elevated unconjugated bilirubin such as hemolytic diseases, congenital hypothyroidism, or Crigler-Najjar or Gilbert syndromes[80], it is important to consider testing for the direct fraction of bilirubin to rule out the possible diagnosis of conjugated hyperbilirubinemia. Prompt diagnosis of certain cholestatic liver diseases, such as biliary atresia is important due to the time sensitivity of efficacious treatment[81][82]. Direct fraction of bilirubin is considered abnormal when the absolute value is over 17 µmol/L[83]. The differential diagnoses, investigations, and treatment of conjugated hyperbilirubinemia are beyond the scope of this statement.
RECOMMENDATIONS:
- Infants with persistent, clinically significant hyperbilirubinemia beyond 14 days of age should be investigated further to rule out urinary tract infections, thyroid disease, hemolytic disease, and inborn errors of metabolism.
- Investigations for prolonged hyperbilirubinemia should include measuring the direct fraction of bilirubin to rule out pathologic cholestasis.
PRE-EXCHANGE TRANSFUSION PROTOCOL AND BLOOD EXCHANGE TRANSFUSION (BET)
Initiating the pre-exchange transfusion protocol
Infants whose TSB is rapidly increasing or nearing the exchange transfusion threshold (Figures 6, 7) based on GA at birth, hours of age, and the presence of neurotoxicity risk factor(s), may need urgent, intensive care to either avoid or perform a BET. Either intervention can prevent the significant neurodevelopmental sequelae of chronic bilirubin encephalopathy (CBE) from kernicterus.
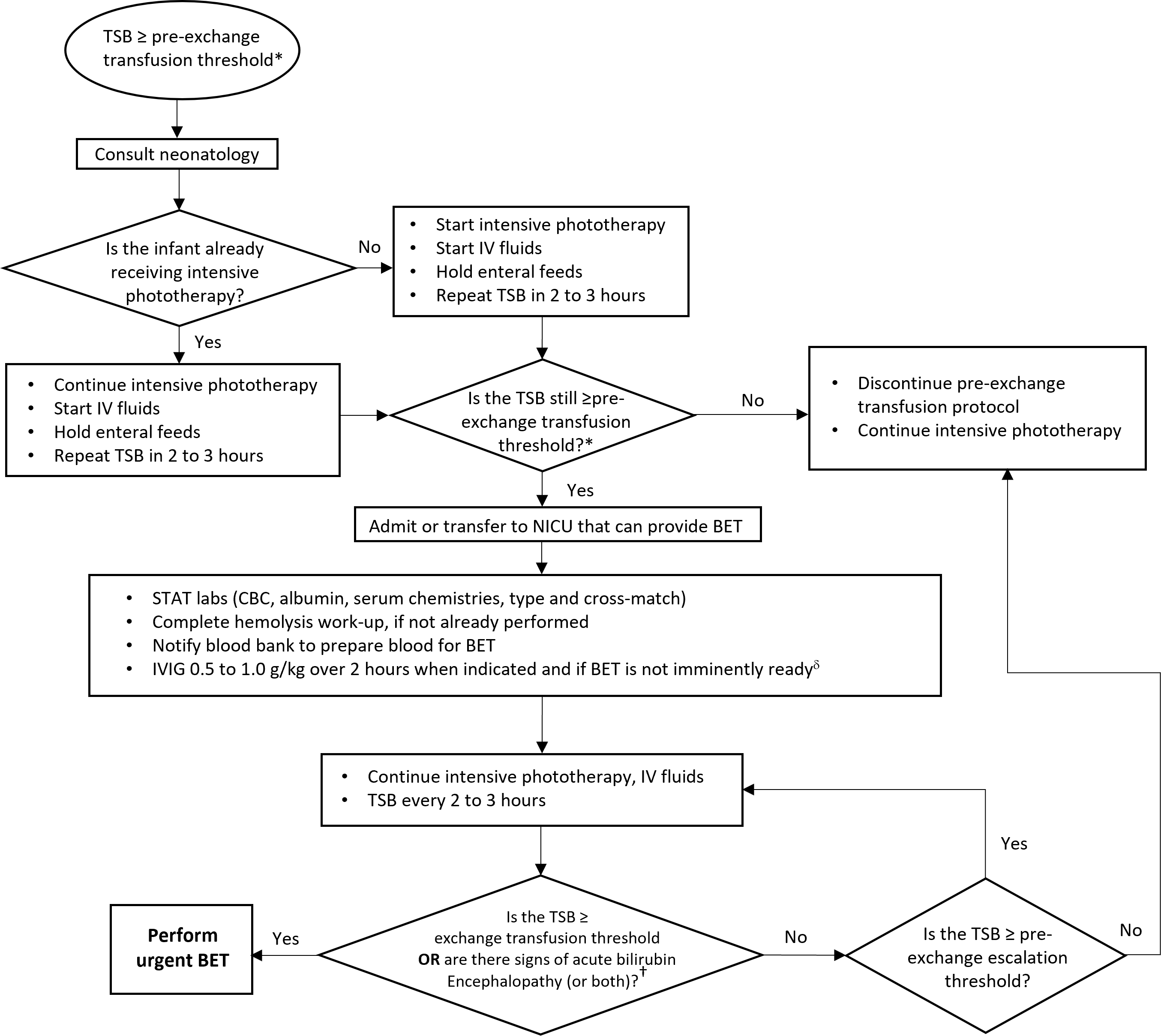
The pre-exchange transfusion protocol is summarized in Figure 5, which outlines the emergent care pathway after an infant meets criteria. The pre-exchange transfusion threshold is defined as a TSB ≤30 µmol/L below the exchange transfusion threshold[12]. Initiating the pre-exchange transfusion protocol is a medical emergency. Start intensive phototherapy without delay, optimally with a fiberoptic pad or blanket to maximize the body area exposed. Consult a neonatologist for guidance on management, including vascular access, withholding feeds in anticipation of a BET, and starting IV fluids.
Urgent blood sampling at initiation of the pre-exchange transfusion protocol includes a total and direct serum bilirubin, CBC, serum albumin, serum chemistries, blood type, and cross-match. Investigations to establish a diagnosis of hemolytic disease should be completed if not already performed. Promptly alert the referral centre’s blood bank that a BET may be needed to prevent delay of treatment while whole blood is reconstituted. The infant’s TSB should be re-measured at least every 2 to 3 hours throughout the transfusion period until the level stabilizes at 30 µmol/L below the exchange transfusion threshold. The infant should be transferred to a neonatal intensive care unit (NICU) or neonatal service that can provide a BET while continuing phototherapy and adjunctive treatments.
RECOMMENDATIONS:
- Infants with hyperbilirubinemia whose TSB level is at or above the pre-exchange transfusion threshold, defined as TSB ≤30 μmol/L below the exchange transfusion threshold (Figures 6, 7), should receive escalated, emergent care based on the pre-exchange transfusion protocol (Figure 5).
- At protocol initiation, order urgent blood-work for: total and direct serum bilirubin, CBC, serum albumin, serum chemistries, blood type, cross-match, and work-up for hemolysis (if not already performed).
- Measure TSB every 2 to 3 hours, and plot levels against the BET thresholds (Figures 6, 7).
- Start intensive phototherapy and IV fluids without delay, and consult with a neonatologist as soon as possible. Withold enteral feeds and prepare for transfer to a NICU where a BET can be provided.
Intravenous immunoglobulin (IVIG)
IVIG is believed to inhibit antibodies that cause red blood cell destruction in conditions such as Rh isoimmunization and other immune-related hemolytic diseases. Results from randomized placebo-controlled trials have been contradictory, which questions the effectiveness of IVIG in reducing the need for and number of BETs in severe hyperbilirubinemia[84]-[86]. IVIG should not be used routinely to treat infants with severe hyperbilirubinemia caused by Rh or ABO antibody-mediated hemolytic disease. Although IVIG’s role in reducing the need for BET is unclear, administering IVIG should be considered in settings where a BET cannot be readily performed[87].
RECOMMENDATION:
- If a BET cannot be readily performed, IV immune globulin (IVIG) should be considered at 0.5 g/kg to 1.0 g/kg over 2 hours for infants with an isoimmune hemolytic disease whose TSB has reached the pre-exchange transfusion threshold. The dose can be repeated in 12 hours.
Figure 5. The pre-exchange transfusion protocol: Steps and sequences
*Pre-exchange transfusion threshold is TSB ≤30 µmol/L of exchange transfusion threshold.
†In infants with isoimmune hemolytic disease whose TSB ≥ pre-exchange transfusion threshold.
§Signs of acute bilirubin encephalopathy (ABE): hypertonia (including opisthotonus and retrocollis), high-pitched cry, fever, irritability or stupor, seizures, coma, or apnea.
BET, Blood exchange transfusion; CBC, Complete blood count; IV, Intravenous; IVIG, Intravenous immunoglobulin; TSB, Total serum bilirubin
Blood exchange transfusion (BET)
When intensive phototherapy and other supportive treatments fail to prevent an infant’s TSB from reaching the GA-specific BET threshold (Figures 6, 7), a double-volume BET should be performed to lower TSB concentrations and minimize risk for bilirubin neurotoxicity. Also, if an infant exhibits any sign(s) of acute bilirubin encephalopathy (ABE), such as hypertonia, opisthotonus, retrocollis, high-pitched cry, fever, irritability, stupor, seizures, coma, or apnea[88], an urgent BET should be performed. Some infants may come to medical attention with a first TSB level at or above the BET threshold. In such cases, initiate intensive phototherapy and IV fluids urgently and repeat TSB in 2 to 3 hours to determine whether a BET is still required (Figure 5).
BET is a procedure with significant morbidity risk and should only be performed in centres with the appropriate expertise, resources, and equipment in place. BET-associated risks include cardiorespiratory instability, catheter-related complications (e.g., thrombosis, infections), hypoglycemia, electrolyte imbalances, thrombocytopenia, coagulopathy, and necrotizing enterocolitis[86][89]. Intensive phototherapy should be restarted following completion of a BET, and TSB levels monitored in accordance with the transfusion protocol (Figure 5).
Blood exchange transfusion thresholds
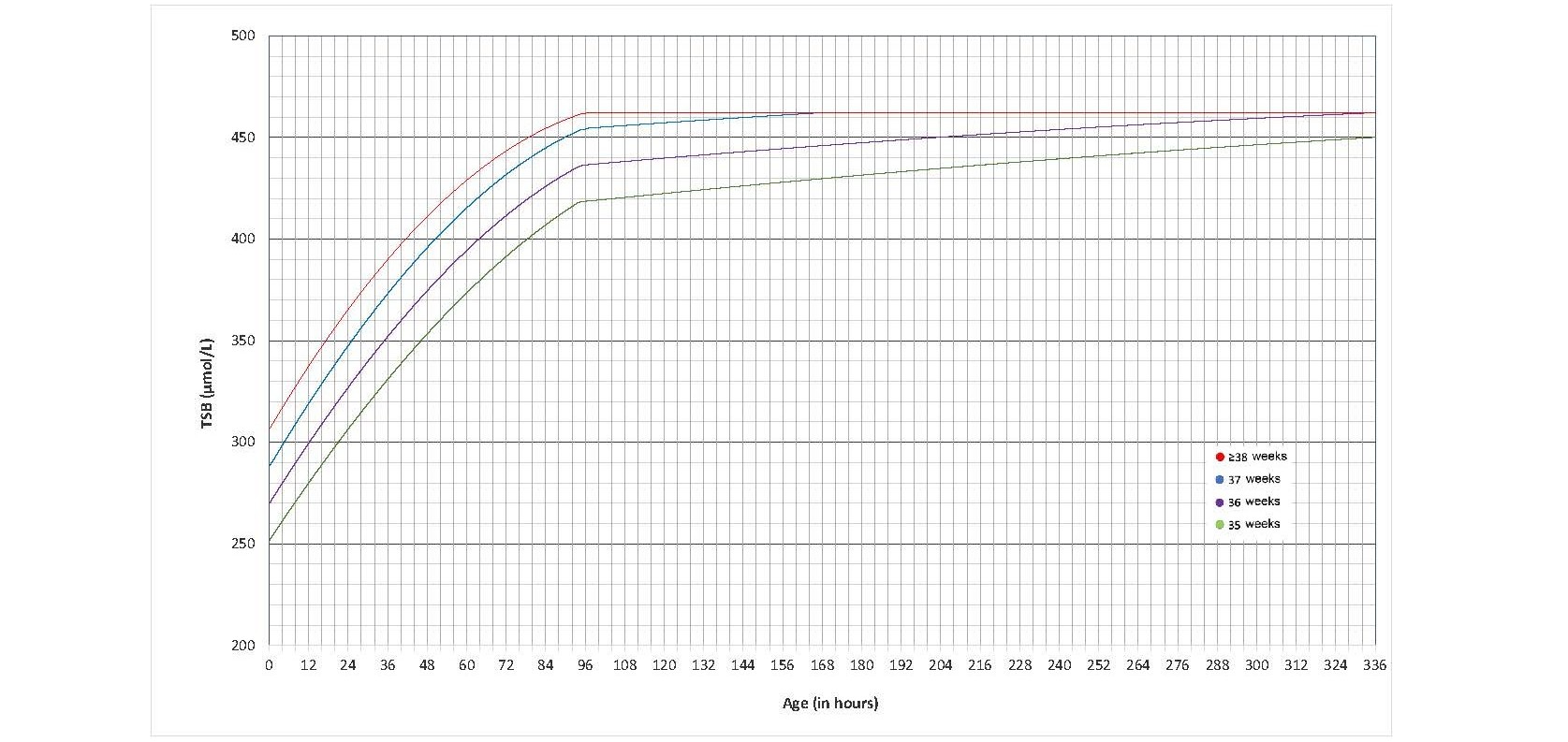
BET thresholds were also revised by the AAP[12] in parallel with changes to phototherapy thresholds (Figures 6 and 7). See Figures S2a-c in the Technical Annex for a visual comparison. While changes from previous BET thresholds were relatively small, there is an important revision to bear in mind. For infants with neurotoxicity risk factor(s), subtracting the treatment threshold by 30 µmol/L approximates the previous exchange transfusion thresholds for high-risk infants. These values coincide with the pre-exchange transfusion threshold defined above, reinforcing the need to be prepared to perform a BET when the jaundiced infant’s TSB is at or above the pre-exchange transfusion threshold.
For infants with neurotoxicity risk factor(s) whose TSB reaches the pre-exchange transfusion threshold (≤30 µmol/L of the exchange transfusion threshold), clinicians may choose to initiate a BET immediately [The fourth rule of 30].
Figure 6. Exchange transfusion thresholds for infants with no neurotoxicity risk factor (data provided by the AAP and used with permission)
Figure 7. Exchange transfusion thresholds for infants with neurotoxicity risk factor(s) (data provided by the AAP and used with permission)
RECOMMENDATIONS:
- A double-volume BET should be performed urgently in infants whose TSB level reaches the exchange transfusion threshold (Figures 6, 7) OR who exhibit signs consistent with acute bilirubin encephalopathy (ABE).
- For infants with neurotoxicity risk factor(s), a BET may be initiated when TSB reaches the pre-exchange transfusion threshold (≤30 µmol/L of the exchange transfusion threshold).
|
Box 1. The ‘rules of 30’ at a glance |
|
First rule: During universal screening, a △TSB≤30 µmol/L requires delay in hospital discharge and close monitoring of TSB. Phototherapy should be considered (Figure 1, Recommendation 4). |
|
Second rule: For infants with neurotoxicity risk factor(s), initiate phototherapy when TSB is ≤30 µmol/L of the phototherapy threshold (Recommendation 12). |
|
Third rule: To minimize risk for rebound hyperbilirubinemia, phototherapy should be discontinued at △TSB >30 µmol/L below treatment threshold for infants born ≥38 weeks GA, and at >60 µmol/L for infants born 35 to 38 weeks GA (Recommendation 16). |
|
Fourth rule: For infants with neurotoxicity risk factor(s), BET may be initiated when TSB reaches the pre-exchange transfusion threshold or ≤30 µmol/L of the exchange transfusion threshold (Recommendation 26). |
|
BET, Blood exchange transfusion; GA, Gestational age; TSB, Total serum bilirubin |
DISCHARGE PLANNING AND FOLLOW-UP
After treatment for hyperbilirubinemia
All infants treated for jaundice should be closely monitored until feeding and weight gain are well established, and TSB levels start to fall. Assessments should include a thorough physical examination with specific focus on any neurological manifestations of bilirubin toxicity (especially in infants whose interventions included a pre-exchange escalation protocol or a BET) and ensuring adequate intake of fluids and nutrition. Infants who had hemolytic jaundice may experience late anemia caused by lingering passive antibodies, with hemoglobulin levels at their lowest point between 4 to 6 weeks in those who did not require a BET, and between 8 to 10 weeks in those who did[90][91].
Bilirubin can affect the brainstem’s cochlear nuclei and the auditory nerve[92]. Cohort studies have shown that the risk for hearing impairment significantly increases in newborns whose TSB level crosses the threshold of 400 µmol/L to 600 µmol/L[93][94]. Their risk increases proportionately with the severity of hyperbilirubinemia and the duration of bilirubin exposure[95][96]. Infants who experience significant hyperbilirubinemia, received a BET, or whose peak TSB reached or exceeded pre-exchange transfusion thresholds should be referred to audiology for a formal hearing assessment[97].
Infants with a history of severe hyperbilirubinemia may have significant, long-term developmental sequelae. Those whose TSB levels reached or exceeded the pre-exchange transfusion threshold, who received a BET, or showed abnormal neurological signs before or after treatment should be referred for longitudinal neurodevelopmental follow-up[98]-[103].
RECOMMENDATIONS:
- Ensure close follow-up following discharge for infants with hyperbilirubinemia secondary to immune-related hemolytic disease, including hemoglobin testing between 4 and 10 weeks of age to identify late anemia.
- Refer infants who experienced significant hyperbilirubinemia (i.e., with peak TSB above 400 μmol/L, or who reached or exceeded the pre-exchange transfusion threshold, or who received a BET) to audiology for a formal hearing assessment.
- Refer infants who reached or exceeded the pre-exchange transfusion threshold with or without receiving a BET, or who showed abnormal neurological signs associated with hyperbilirubinemia, for longitudinal neurodevelopmental follow-up.
CONCLUSION
Kernicterus caused by severe hyperbilirubinemia is a preventable condition in newborns. Detecting jaundice early and acting promptly, providing anticipatory guidance to families on signs and symptoms, and ensuring appropriate postnatal follow-up and lactation support in-community can help prevent adverse neurodevelopmental sequelae from severe hyperbilirubinemia[104]. This statement recommends key strategies for identifying newborns at high risk for developing significant hyperbilirubinemia and standardizing treatment. From a systems perspective, collaborating with community health providers and laboratories, providing breastfeeding support, and partnering with families are essential to preventing and managing neonatal hyperbilirubinemia.
Tools for Practitioners
Individual graphs of phototherapy and blood exchange transfusion thresholds for each gestational age group as listed in Figures 2, 3, 6, and 7 have been created to allow clinicians to print and use to follow each patient’s TSB or TcB trend. Follow the link to access these printable graphs.
An online tool, called hyperbili has been developed for neonatal providers to aid in management of neonatal hyperbilirubinemia based on this position statement. The tool can be accessed here. Our thanks to Dr. Michael Hill for developing it.
Acknowledgements
The authors would like to thank Drs. Michael Sgro, Douglas Campbell, Lani Lieberman, and Drs. Alex Kemper and Thomas Newman from the AAP for their advice and guidance while developing this statement. The document was reviewed by the Acute Care, Community Paediatrics, and Nutrition and Gastroenterology Committees of the Canadian Paediatric Society, and by members of the College of Family Physicians of Canada, Maternity Interest Group.
CANADIAN PAEDIATRIC SOCIETY FETUS AND NEWBORN COMMITTEE (2024-2025)
Members: Michael Narvey MD (Chair), Heidi Budden MD (Board Representative), Souvik Mitra MD MSC, Eugene Ng MD, Gabriel Altit MD, Nicole Radziminski MD, Anne-Sophie Gervais MD (Resident Member)
Liaisons: William Ehman (College of Family Physicians of Canada), Chantal Nelson (Public Health Agency of Canada), Eric Eichenwald (American Academy of Pediatrics, Committee on Fetus & Newborn), Douglas Wilson (The Society of Obstetricians and Gynaecologists of Canada), Isabelle Milette (Canadian Association of Neonatal Nurses), Emer Finan MBBCH (CPS Neonatal-Perinatal Medicine Section)
Authors: Eugene Ng MD, Gabriel Altit MD, Chloe Joynt MD, Nicole Radziminski MD, Michael Narvey MD
Funding
The authors have no funding to declare.
Conflict of Interest Disclosure
The authors have indicated they have no conflicts of interest.
References
- Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and rhesus disease of the newborn: Incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res 2013;74 (Suppl 1):86-100. doi: 10.1038/pr.2013.208
- Zhang M, Tang J, He Y, et al. Systematic review of global clinical practice guidelines for neonatal hyperbilirubinemia. BMJ Open 2021;11(1):e040182. doi: 10.1136/bmjopen-2020-040182
- Barrington KJ, Sankaran K; Canadian Paediatric Society, Fetus and Newborn Committee. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation): Summary. Paediatr Child Health 2007;12(5):401-18. doi: 10.1093/pch/12.5.401
- Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ 2006;175(6):587-90. doi: 10.1503/cmaj.060328
- Sgro M, Kandasamy S, Shah V, Ofner M, Campbell D. Severe neonatal hyperbilirubinemia decreased after the 2007 Canadian guidelines. J Pediatr 2016;171:43-7. doi: 10.1016/j.jpeds.2015.12.067
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297-316. doi: 10.1542/peds.114.1.297
- AlOtaibi SF, Blaser S, MacGregor DL. Neurological complications of kernicterus. Can J Neurol Sci 2005;32(3):311-5. doi: 10.1017/s0317167100004182
- Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics 1995;96(4 Pt 1):730-3. doi: 10.1542/peds.96.4.730
- Manning D, Todd P, Maxwell M, Jane Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed 2007;92(5):F342-6. doi: 10.1136/adc.2006.105361
- Newman TB, Maisels MJ. Less aggressive treatment of neonatal jaundice and reports of kernicterus: Lessons about practice guidelines. Pediatrics 2000;105(1 Pt 3):242-5. doi: 10.1542/peds.105.S2.242
- Sgro M, Campbell DM, Kandasamy S, Shah V. Incidence of chronic bilirubin encephalopathy in Canada, 2007-2008. Pediatrics 2012;130(4):e886-90. doi: 10.1542/peds.2012-0253
- Kemper AR, Newman TB, Slaughter JL, et al. Clinical practice guideline revision: Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2022;150(3):e2022058859. doi: 10.1542/peds.2022-058859
- Fung-Kee-Fung K, Wong K, Walsh J, Hamel C, Clarke G. Guideline no. 448: Prevention of Rh D alloimmunization. J Obstet Gynaecol Can 2024;46(4):102449. doi: 10.1016/j.jogc.2024.102449
- AlKhater SA, Albalwi RA, Alomar SA, et al. Value of the direct antiglobulin test in predicting the need for phototherapy in newborns. J Blood Med 2021;12:53-61. doi: 10.2147/JBM.S291606
- Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health 2005;41(9-10):504-7. doi: 10.1111/j.1440-1754.2005.00692.x
- Herschel M, Karrison T, Wen M, Caldarelli L, Baron B. Evaluation of the direct antiglobulin (Coombs’) test for identifying newborns at risk for hemolysis as determined by end-tidal carbon monoxide concentration (ETCOc); and comparison of the Coombs’ test with ETCOc for detecting significant jaundice. J Perinatol 2002;22(5):341-7. doi: 10.1038/sj.jp.7210702
- Meberg A, Johansen KB. Screening for neonatal hyperbilirubinaemia and ABO alloimmunization at the time of testing for phenylketonuria and congenital hypothyreosis. Acta Paediatr 1998;87(12):1269-74. doi: 10.1080/080352598750030960
- Anderson NB, Calkins KL. Neonatal indirect hyperbilirubinemia. Neoreviews 2020;21(11):e749-60. doi: 10.1542/neo.21-11-e749
- Maayan-Metzger A, Schwartz T, Sulkes J, Merlob P. Maternal anti-D prophylaxis during pregnancy does not cause neonatal haemolysis. Arch Dis Child Fetal Neonatal Ed 2001;84(1):F60-2. doi: 10.1136/fn.84.1.f60
- Keir A, Agpalo M, Lieberman L, Callum J. How to use: The direct antiglobulin test in newborns. Arch Dis Child Educ Pract Ed 2015;100(4):198-203. doi: 10.1136/archdischild-2013-305553
- Sgro M, Campbell D, Shah V. Coombs’ testing and neonatal hyperbilirubinemia. CMAJ 2007;176(7):973-6. doi: 10.1503/cmaj.1060213
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103(1):6-14. doi: 10.1542/peds.103.1.6
- Kuzniewicz MW, Park J, Niki H, Walsh EM, McCulloch CE, Newman TB. Predicting the need for phototherapy after discharge. Pediatrics 2021;147(5):e2020019778. doi: 10.1542/peds.2020-019778
- Stevenson DK, Fanaroff AA, Maisels MJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. J Perinatol 2001;21(Suppl 1):S63-72; discussion S83-7. doi: 10.1038/sj.jp.7210638
- Mallard C, Wang X. Infection-induced vulnerability of perinatal brain injury. Neurol Res Int 2012;2012:102153. doi: 10.1155/2012/102153
- Brito MA, Palmela I, Cardoso FL, Sá-Pereira I, Brites D. Blood-brain barrier and bilirubin: Clinical aspects and experimental data. Arch Med Res 2014;45(8):660-76. doi: 10.1016/j.arcmed.2014.11.015
- Ahlfors CE. The Bilirubin Binding Panel: A Henderson-Hasselbalch approach to neonatal hyperbilirubinemia. Pediatrics 2016;138(4):e20154378. doi: 10.1542/peds.2015-4378
- Maisels MJ, Newman TB. Jaundice in full-term and near-term babies who leave the hospital within 36 hours. The pediatrician's nemesis. Clin Perinatol 1998;25(2):295-302. doi: 10.1016/S0095-5108(18)30116-7
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013(7):CD004074. doi: 10.1002/14651858.CD004074.pub3
- Yaşartekin Y, Sarıcı SU, Özcan M, et al. Investigation of the relationship between cord clamping time and risk of hyperbilirubinemia. Turk J Pediatr 2020;62(5):756-62. doi: 10.24953/turkjped.2020.05.006
- Kuzniewicz MW, Escobar GJ, Wi S, Liljestrand P, McCulloch C, Newman TB. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A nested case-control study. J Pediatr 2008;153(2):234-40. doi:10.1016/j.jpeds.2008.01.028
- Maisels MJ, Watchko JF. Improving post-discharge neonatal surveillance for the jaundiced newborn. Acta Paediatr 2020;109(5):872-3. doi: 10.1111/apa.15154
- Flaherman VJ, Maisels MJ; Academy of Breastfeeding Medicine. ABM clinical protocol #22: Guidelines for management of jaundice in the breastfeeding infant 35 weeks or more of gestation—revised 2017. Breastfeed Med 2017;12(5):250-7. doi: 10.1089/bfm.2017.29042.vjf
- Huang A, Tai BC, Wong LY, Lee J, Yong EL. Differential risk for early breastfeeding jaundice in a multi-ethnic Asian cohort. Ann Acad Med Singap 2009;38(3):217-24. doi: 10.47102/annals-acadmedsg.V38N3p217
- Sato H, Uchida T, Toyota K, et al. Association of neonatal hyperbilirubinemia in breast-fed infants with UGT1A1 or SLCOs polymorphisms. J Hum Genet 2015;60(1):35-40. doi: 10.1038/jhg.2014.98
- Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. J Pediatr 2002;140(4):396-403. doi: 10.1067/mpd.2002.123098
- Maisels MJ, Gifford K. Breast-feeding, weight loss, and jaundice. J Pediatr 1983;102(1):117-8. doi: 10.1016/s0022-3476(83)80305-9
- Chen YJ, Yeh TF, Chen CM. Effect of breast‐feeding frequency on hyperbilirubinemia in breast‐fed term neonate. Pediatr Int 2015;57(6):1121-5. doi: 10.1111/ped.12667
- Hassan B, Zakerihamidi M. The correlation between frequency and duration of breastfeeding and the severity of neonatal hyperbilirubinemia. J Mat Fetal Neonat Med 2018;31(4):457-63. doi: 10.1080/14767058.2017.1287897
- Chang RJ, Chou HC, Chang YH, et al. Weight loss percentage prediction of subsequent neonatal hyperbilirubinemia in exclusively breastfed neonates. Pediatr Neonatol 2012;53(1):41-4. doi: 10.1016/j.pedneo.2011.11.008
- Chen YJ, Chen WC, Chen CM. Risk factors for hyperbilirubinemia in breastfed term neonates. Eur J Pediatr 2012;171(1):167-71. doi: 10.1007/s00431-011-1512-8
- Tomlinson C, Haiek LN; Canadian Paediatric Society, Nutrition and Gastroenterology Committee. Breastfeeding and human milk in the NICU: From birth to discharge. June 6, 2023.
- Maisels MJ, Kring EA, Coffey MP. Heme catabolism and bilirubin production in readmitted jaundiced newborns. J Pediatr 2020;226:285-8. doi: 10.1016/j.jpeds.2020.06.012
- Keren R, Tremont K, Luan X, Cnaan A. Visual assessment of jaundice in term and late preterm infants. Arch Dis Child Fetal Neonatal Ed 2009;94(5):F317-22. doi: 10.1136/adc.2008.150714
- Eidelman AI, Schimmel MS, Algur N, Eylath U. Capillary and venous bilirubin values: They are different--and how! Am J Dis Child 1989;143(6):642. doi: 10.1001/archpedi.1989.02150180020007
- Leslie GI, Philips JB, Cassady G. Capillary and venous bilirubin values. Are they really different? Am J Dis Child 1987;141(11):1199-200. doi: 10.1001/archpedi.1987.04460110069024
- Maisels MJ. Capillary vs venous bilirubin values. Am J Dis Child 1990;144(5):521-2. doi: 10.1001/archpedi.1990.02150290015011
- Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: An update with clarifications. Pediatrics 2009;124(4):1193-8. doi: 10.1542/peds.2009-0329
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106(2):E17. doi: 10.1542/peds.106.2.e17
- Campbell DM, Danayan KC, McGovern V, Cheema S, Stade B, Sgro M. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health 2011;16(3):141-5. doi: 10.1093/pch/16.3.141
- Engle WD, Jackson GL, Stehel EK, Sendelbach DM, Manning MD. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J Perinatol 2005;25(7):486-90. doi: 10.1038/sj.jp.7211333
- Dam-Vervloet AJ, van Erk MD, Doorn N, et al. Inter-device reproducibility of transcutaneous bilirubin meters. Pediatr Res 2021;89(4):770-5. doi: 10.1038/s41390-020-01118-6
- Ng Y, Maul T, Viswanathan S, Chua C. The accuracy of transcutaneous bilirubin as a screening test in preterm infants. Cureus 2023;15(8):e42793. doi: 10.7759/cureus.42793
- Rodríguez-Capote K, Kim K, Paes B, Turner D, Grey V. Clinical implication of the difference between transcutaneous bilirubinometry and total serum bilirubin for the classification of newborns at risk of hyperbilirubinemia. Clin Biochem 2009;42(3):176-9. doi: 10.1016/j.clinbiochem.2008.09.108
- Borden AR, Satrom KM, Wratkowski P, et al. Variation in the phototherapy practices and irradiance of devices in a major metropolitan area. Neonatology 2018;113(3):269-74. doi: 10.1159/000485369
- Sampurna MTA, Etika R, Utomo MT, et al. An evaluation of phototherapy device performance in a tertiary health facility. Heliyon 2020;6(9):e04950. doi: 10.1016/j.heliyon.2020.e04950
- Slaughter JL, Kemper AR, Newman TB. Technical report: Diagnosis and management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2022;150(3): e2022058865. doi: 10.1542/peds.2022-058865
- Ng E. Screening for neonatal hyperbilirubinemia: Translating guidelines into practice. Neonatology Today 2010;5(3):1-6.
- Gourley GR. Breast-feeding, neonatal jaundice and kernicterus. Semin Neonatol 2002;7(2):135-41. doi: 10.1053/siny.2002.0101
- Boo NY, Lee HT. Randomized controlled trial of oral versus intravenous fluid supplementation on serum bilirubin level during phototherapy of term infants with severe hyperbilirubinaemia. J Paediatr Child Health 2002;38(2):151-5. doi: 10.1046/j.1440-1754.2002.00746.x
- Lai NM, Ahmad Kamar A, Choo YM, Kong JY, Ngim CF. Fluid supplementation for neonatal unconjugated hyperbilirubinaemia. Cochrane Database Syst Rev 2017;8(8):CD011891. doi: 10.1002/14651858.CD011891.pub2
- Nicoll A, Ginsburg R, Tripp JH. Supplementary feeding and jaundice in newborns. Acta Paediatr Scand 1982;71(5):759-61. doi: 10.1111/j.1651-2227.1982.tb09515.x
- Hannon PR, Willis SK, Scrimshaw SC. Persistence of maternal concerns surrounding neonatal jaundice: An exploratory study. Arch Pediatr Adolesc Med 2001;155(12):1357-63. doi: 10.1001/archpedi.155.12.1357
- Szucs KA, Rosenman MB. Family-centered, evidence-based phototherapy delivery. Pediatrics 2013;131(6):e1982-5. doi: 10.1542/peds.2012-3479
- Wang J, Guo G, Li A, Cai WQ, Wang X. Challenges of phototherapy for neonatal hyperbilirubinemia (Review). Exp Ther Med 2021;21(3):231. doi: 10.3892/etm.2021.9662
- Altit G, Hamilton D, O’Brien K; Canadian Paediatric Society, Fetus and Newborn Committee. Skin-to-skin care (SSC) for term and preterm infants. January 11, 2024.
- Kaplan M, Maisels MJ. Natural history of early neonatal bilirubinemia: A global perspective. J Perinatol 2021;41(4):873-8. doi: 10.1038/s41372-020-00901-x
- WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. Bull World Health Organ 1989;67(6):601-11.
- Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol 2009;29(Suppl 1):S25-45. doi: 10.1038/jp.2008.211
- Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics 2014;134(3):504-9. doi: 10.1542/peds.2014-0987
- Ostfeld-Johns S, Aragona E, Hart L. Removing race from hyperbilirubinemia guidelines is not enough. JAMA Pediatr 2022;176(12):1163-4. doi: 10.1001/jamapediatrics.2022.3865
- Barak M, Berger I, Dollberg S, Mimouni FB, Mandel D. When should phototherapy be stopped? A pilot study comparing two targets of serum bilirubin concentration. Acta Paediatr 2009;98(2):277-81. doi: 10.1111/j.1651-2227.2008.01015.x
- Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics 2019;144(1):e20183712. doi: 10.1542/peds.2018-3712
- Almohammadi H, Nasef N, Al-Harbi A, Saidy K, Nour I. Risk factors and predictors of rebound hyperbilirubinemia in a term and late-preterm infant with hemolysis. Am J Perinatol 2022;39(8):836-43. doi: 10.1055/s-0040-1718946
- Kaplan M, Kaplan E, Hammerman C, et al. Post-phototherapy neonatal bilirubin rebound: A potential cause of significant hyperbilirubinaemia. Arch Dis Child 2006;91(1):31-4. doi: 10.1136/adc.2005.081224
- Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med 2002;156(7):669-72. doi: 10.1001/archpedi.156.7.669
- Tan KL, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr 2003;92(3):327-31. doi: 10.1111/j.1651-2227.2003.tb00554.x
- Grabenhenrich J, Grabenhenrich L, Bührer C, Berns M. Transcutaneous bilirubin after phototherapy in term and preterm infants. Pediatrics 2014;134(5):e1324-9. doi: 10.1542/peds.2014-1677
- Rennie J, Burman-Roy S, Murphy MS; Guideline Development Group. Neonatal jaundice: Summary of NICE guidance. BMJ 2010;340:c2409. doi: 10.1136/bmj.c2409
- Pillai A, Pandita A, Osiovich H, Manhas D. Pathogenesis and management of indirect hyperbilirubinemia in preterm neonates less than 35 weeks: Moving toward a standardized approach. Neoreviews 2020;21(5):e298-e307. doi: 10.1542/neo.21-5-e298
- Giannattasio A, Ranucci G, Raimondi F. Prolonged neonatal jaundice. Ital J Pediatr 2015;41(Suppl 2):A36. doi: 10.1186/1824-7288-41-S2-A36
- Venigalla S, Gourley GR. Neonatal cholestasis. Semin Perinatol 2004;28(5):348-55. doi: 10.1053/j.semperi.2004.09.008
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: Joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017;64(1):154-68. doi: 10.1097/MPG.0000000000001334
- Santos MC, Sá C, Gomes SC, Camacho LA, Moreira ME. The efficacy of the use of intravenous human immunoglobulin in Brazilian newborns with rhesus hemolytic disease: A randomized double-blind trial. Transfusion 2013;53(4):777-82. doi: 10.1111/j.1537-2995.2012.03827.x
- Smits-Wintjens VE, Walther FJ, Rath ME, et al. Intravenous immunoglobulin in neonates with rhesus hemolytic disease: A randomized controlled trial. Pediatrics 2011;127(4):680-6. doi: 10.1542/peds.2010-3242
- Zwiers C, Scheffer-Rath ME, Lopriore E, de Haas M, Liley HG. Immunoglobulin for alloimmune hemolytic disease in neonates. Cochrane Database Syst Rev 2018;3(3):CD003313. doi: 10.1002/14651858.CD003313.pub2
- Lieberman L, Lopriore E, Baker JM, et al. International guidelines regarding the role of IVIG in the management of Rh- and ABO-mediated haemolytic disease of the newborn. Br J Haematol 2022;198(1):183-95. doi: 10.1111/bjh.18170
- Sgro M, Campbell D, Barozzino T, Shah V. Acute neurological findings in a national cohort of neonates with severe neonatal hyperbilirubinemia. J Perinatol 2011;31(6):392-6. doi: 10.1038/jp.2010.137
- Pace EJ, Brown CM, DeGeorge KC. Neonatal hyperbilirubinemia: An evidence-based approach. J Fam Pract 2019;68(1):E4-E11.
- Mitchell S, James A. Severe late anemia of hemolytic disease of the newborn. Paediatr Child Health 1999;4(3):201-3. doi: 10.1093/pch/4.3.201
- Ebbesen F. Late anaemia in infants with rhesus haemolytic disease treated with intensive phototherapy. Eur J Pediatr 1979;130(4):285-90. doi: 10.1007/BF00441365
- Olds C, Oghalai JS. Bilirubin-induced audiologic injury in preterm infants. Clin Perinatol 2016;43(2):313-23. doi: 10.1016/j.clp.2016.01.006
- Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal phototherapy and infantile cancer. Pediatrics 2016;137(6):e20151353. doi:10.1542/peds.2015-1353
- Dey SK, Islam S, Jahan I, et al. Association of hyperbilirubinemia requiring phototherapy or exchange transfusion with hearing impairment among admitted term and late preterm newborn in a NICU. Mymensingh Med J 2020;29(2):405-13.
- Akinpelu OV, Waissbluth S, Daniel SJ. Auditory risk of hyperbilirubinemia in term newborns: A systematic review. Int J Pediatr Otorhinolaryngol 2013;77(6):898-905. doi: 10.1016/j.ijporl.2013.03.029
- Besli GE, Metin F, Aksit MA, Saltik S. Long-term effects of indirect hyperbilirubinemia on auditory and neurological functions in term newborns. Medeni Med J 2020;35(1):29-39. doi: 10.5222/MMJ.2020.26986
- Patel H, Feldman M. Universal newborn hearing screening. Paediatr Child Health 2011;16(5):301-10. doi: 10.1093/pch/16.5.301
- Lunsing RJ, Pardoen WF, Hadders-Algra M. Neurodevelopment after moderate hyperbilirubinemia at term. Pediatr Res 2013;73(5):655-60. doi: 10.1038/pr.2013.28
- Grimmer I, Berger-Jones K, Bührer C, Brandl U, Obladen M. Late neurological sequelae of non-hemolytic hyperbilirubinemia of healthy term neonates. Acta Paediatr 1999;88(6):661-3. doi: 10.1080/08035259950169341
- Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: Another look at the Collaborative Perinatal Project. Pediatrics 1993;92(5):651-7. doi: 10.1542/peds.92.5.651
- Ozmert E, Erdem G, Topçu M, et al. Long-term follow-up of indirect hyperbilirubinemia in full-term Turkish infants. Acta Paediatr 1996;85(12):1440-4. doi: 10.1111/j.1651-2227.1996.tb13949.x
- Soorani-Lunsing I, Woltil HA, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates really safe for the brain? Pediatr Res 2001;50(6):701-5. doi: 10.1203/00006450-200112000-00012
- Hokkanen L, Launes J, Michelsson K. Adult neurobehavioral outcome of hyperbilirubinemia in full term neonates—A 30 year prospective follow-up study. Peer J 2014;2:e294. doi: 10.7717/peerj.294
- Manning D. Neonatal jaundice: In the eye of the beholder? Arch Dis Child Fetal Neonatal Ed 2009;94(5):F314-6. doi: 10.1136/adc.2008.156356
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.