Position statement
Management of the paediatric patient with acute head trauma
Posted: Mar 28, 2025
Principal author(s)
Kevin Chan, Catherine A. Farrell, Laurel Chauvin-Kimoff; Canadian Paediatric Society, Acute Care Committee
Abstract
Acute head trauma (AHT) leading to traumatic brain injury is an important cause of paediatric morbidity and mortality. Injury severity depends on the mechanism of trauma and age of the child. The vast majority of childhood AHT cases are mild, require no therapy, and leave no long-term sequelae. However, it is important to identify individuals at risk for significant injury and those who require specific evaluation and intervention. This statement replaces a 2013 document from the Canadian Paediatric Society on this topic. It describes issues related to AHT in infants, children, and youth, including clinical manifestations, initial management priorities, guidelines for observation, imaging, and subsequent follow-up and treatment. The evaluation of patients with AHT at the time of initial assessment is also addressed.
Keywords: Acute head trauma (AHT); Computed tomography (CT) scan; Prevention; Skull x-rays; Traumatic brain injury (TBI)
Box 1. Acronyms
|
AHT |
Acute head trauma |
|
CABCD-E |
Catastrophic bleeding, airway, breathing, circulation, disability, and exposure |
|
CATCH |
Canadian Assessment of Tomography for Childhood Head Injury |
|
CHALICE |
Children’s Head injury ALgorithm for the prediction of Important Clinical Events |
|
ciTBI |
Clinically important traumatic brain injury |
|
CT |
Computed tomography |
|
ED |
Emergency department |
|
GCS |
Glasgow Coma Scale |
|
ICI |
Intracranial injury |
|
ICP |
Intracranial pressure |
|
LOC |
Level of consciousness |
|
MRI |
Magnetic resonance imaging |
|
PECARN |
Pediatric Emergency Care Applied Research Network |
|
TBI |
Traumatic brain injury |
|
THI-CM |
Traumatic head injury due to child maltreatment |
Acute head trauma (AHT) leading to traumatic brain injury (TBI) is an important cause of morbidity and mortality in childhood in developed countries, and a common presentation to the emergency department (ED)[1]. The incidence of AHT in paediatric patients varies according to definition and methodology. However, the estimated global annual rate for AHT ranges between 47 to 280 cases per 100,000 population[1]. Among children younger than 14 years of age in the United States, there are 500,000 emergency department (ED) visits, 37,000 hospitalizations, and 2000 deaths annually[2].
The most common causes of head trauma in children and youth presenting to Canadian EDs are[3]:
- Falls
- Sport-related injuries
- Being hit on the head by an object or colliding with an obstacle
- Injuries involving the use of a bicycle
- Injuries involving motor vehicles, especially as a pedestrian
Only a small proportion of children with AHT will have TBI. TBI refers to the symptoms and signs from the trauma to the brain itself, with or without accompanying findings on imaging studies. Clinically important traumatic brain injury (ciTBI) is defined by the evidence of an intracranial injury (ICI) on imaging (e.g., subdural hematoma, epidural hematoma, or cerebral contusion) with one or more of the following: requirement for neurosurgical intervention, endotracheal intubation for the management of head injury, hospitalization for 48 hours or longer, or death[4].
Concussion is a form of TBI induced by biomechanical forces that result in signs and symptoms of neurological impairment that typically resolve spontaneously within 4 weeks of injury[5]. However, there may be persistent symptoms and impaired functioning for a length of time following the initial event[6].
The unique anatomy of children increases their risk for an ICI with head trauma, with risk factors including a larger head-to-body size ratio, thinner cranial bones, and less myelinated neural tissue[7]. ICI is more frequent following falls from a height greater than three feet (91 cm or twice the length/height of the individual), involvement in a motor vehicle collision (either as a passenger or pedestrian), or following a high-velocity projectile impact[8]. Paediatric patients with TBI more commonly develop a pattern of diffuse axonal injury and secondary cerebral edema, compared with adults[9]. AHT requiring neurosurgical intervention is rare (0.14%)[4].
Clinical manifestations
Children with TBI present with a variety of symptoms, including:
- Headache
- Nausea and vomiting
- Loss of consciousness
- Impaired LOC, disorientation or confusion
- Amnesia
- Blurred vision
- Seizures
Signs of concern for ciTBI include:
- Palpable skull fracture
- Signs of basilar skull fracture (raccoon eyes, hemotympanum)
- Occipital, parietal, or temporal scalp hematoma
- Increasing lethargy and decreasing LOC
Younger children may present with lethargy or irritability. No single clinical symptom or sign is diagnostic of ICI, but the presence of a constellation of symptoms and signs makes ICI more likely and requires a computed tomography (CT) scan.
The Glasgow Coma Scale (GCS) is a validated tool used to evaluate level of consciousness[10][11]. The Paediatric GCS has been shown to be particularly useful in preverbal children[12][13].
Classification of the severity of TBI
For the purposes of this statement, TBI is classified according to GCS as follows:
GCS 14 to 15: Minor TBI
GCS 9 to 13: Moderate TBI
GCS <8: Severe TBI
Most paediatric patients presenting for medical assessment for possible head injury have a minor TBI. Patients with moderate or severe TBI are more likely to have intracranial pathology and require either supportive care or specific treatment in a hospital setting.
Initial management priorities
The first priority is to stabilize the patient as required. In the setting of moderate to severe TBI, avoid hypoxia, maintain cerebral perfusion pressure, and avoid secondary injury to the traumatized brain. A structured approach to the assessment of catastrophic bleeding, airway, breathing, circulation, disability, and exposure (CABCD-E) is advised[14].
Remember that vital signs may be unstable in severe TBI. Early and definitive treatment of a primary ICI may be required in cases of high risk for immediate brain herniation.
A pertinent history should be obtained. Elements to include are:
- The mechanism of head trauma, and whether the event was witnessed or not
- The state in which the patient was found, including LOC, seizures, or focal neurological signs
- Presenting symptoms, especially ongoing impaired (or decreasing) level of consciousness, disorientation or confusion, amnesia, worsening headache, or vomiting
- Medical history of prior head injury, neurological disorders, medication use, allergies, and bleeding diathesis.
Traumatic head injury due to child maltreatment (THI-CM) should be considered, especially in situations of altered level of consciousness without obvious cause, or when the clinical findings are not compatible with the history provided[15]. THI-CM may not be recognized initially due to variable modes of presentation and the typically young age of victims[16]. Delays in recognizing TBI in this context can lead to relatively poor outcomes.
The extent of investigations, including cerebral imaging, and the need for observation, hospitalization, or specific intervention, largely depends on the clinical state of the patient at the time of initial assessment[15].
Imaging
Indications for a CT scan
All patients presenting with moderate or severe head trauma (GCS <13) should undergo a CT head scan. However, there is considerable debate around which minor head trauma (GCS 14 to 15) requires a CT, based on the potential for late deterioration resulting from delayed diagnosis of an ICI and the relative unreliability of clinical signs in predicting ICI[17]. The low rate of positive findings on CT scan, the need to sedate some patients before performing the examination, and concern about the risk of radiation exposure[18][19] have prompted the development of clinical prediction rules to guide clinicians in deciding for whom a scan should be performed. These rules involve some combination of variables based on the mechanism of trauma, signs and symptoms on initial assessment, or status after a period of observation.
There are three large studies:
- Pediatric Emergency Care Applied Research Network (PECARN), from the US, included 42,412 patients from 25 sites[4]. This group’s approach differed from previous studies in identifying elements whose absence would reduce the need for a CT scan. While highly sensitive (100% in children <2 years old and 96.8% in children >2 years old) and relatively specific (53.7% in children <2 years-old and 59.8% in children >2 years old)[4], widespread application of these algorithms may lead to greater use of CT scans[20].
- The Canadian Assessment of Tomography for Childhood Head injury (CATCH) rule[3] was a prospective cohort study involving 3866 children presenting with symptomatic minor head trauma to ten Canadian paediatric teaching institutions. A prospective validation and refinement of the rule (CATCH2) included >4 episodes of vomiting and was found to be 99.5% sensitive (95% CI 97 to 100) and 47.8% specific (95% CI 46.8 to 49.4) for predicting acute brain injury and would require that 55% of patients undergo CT[21].
- The Children’s Head injury ALgorithm for the prediction of Important Clinical Events (CHALICE) in the United Kingdom[8] included 22,772 children from ten hospitals in northwest England. Their study was 98% sensitive (95% CI 96 to 100) and 87% specific (95% CI 86 to 87) and would require a CT scan rate of 14%.
There have been a number of studies comparing the three rules, and while all three provide similar results, there has been a marginal preference for the PECARN head injury rule because of higher sensitivity and specificity for children <2 years of age[22][23] (Grade B, level of evidence II), and the fact that the PECARN head injury rule was the first to be prospectively validated in multiple settings. The PECARN rule was derived to have high sensitivity for whom not to scan, but the algorithm is not definitive regarding which children should receive CT scans. PECARN’s head injury rule has been adopted in the latest iteration of Advanced Trauma Life Support (ATLS) (10th edition)[24].
The challenge remains for many in the intermediate risk group, and shared decision-making should depend on signs and symptoms, progression or regression of symptoms, and the comfort of the caregiver. These guidelines are intended to assist decision-making. MD+Calc is a useful tool to guide the application of the PECARN head injury rules[25].
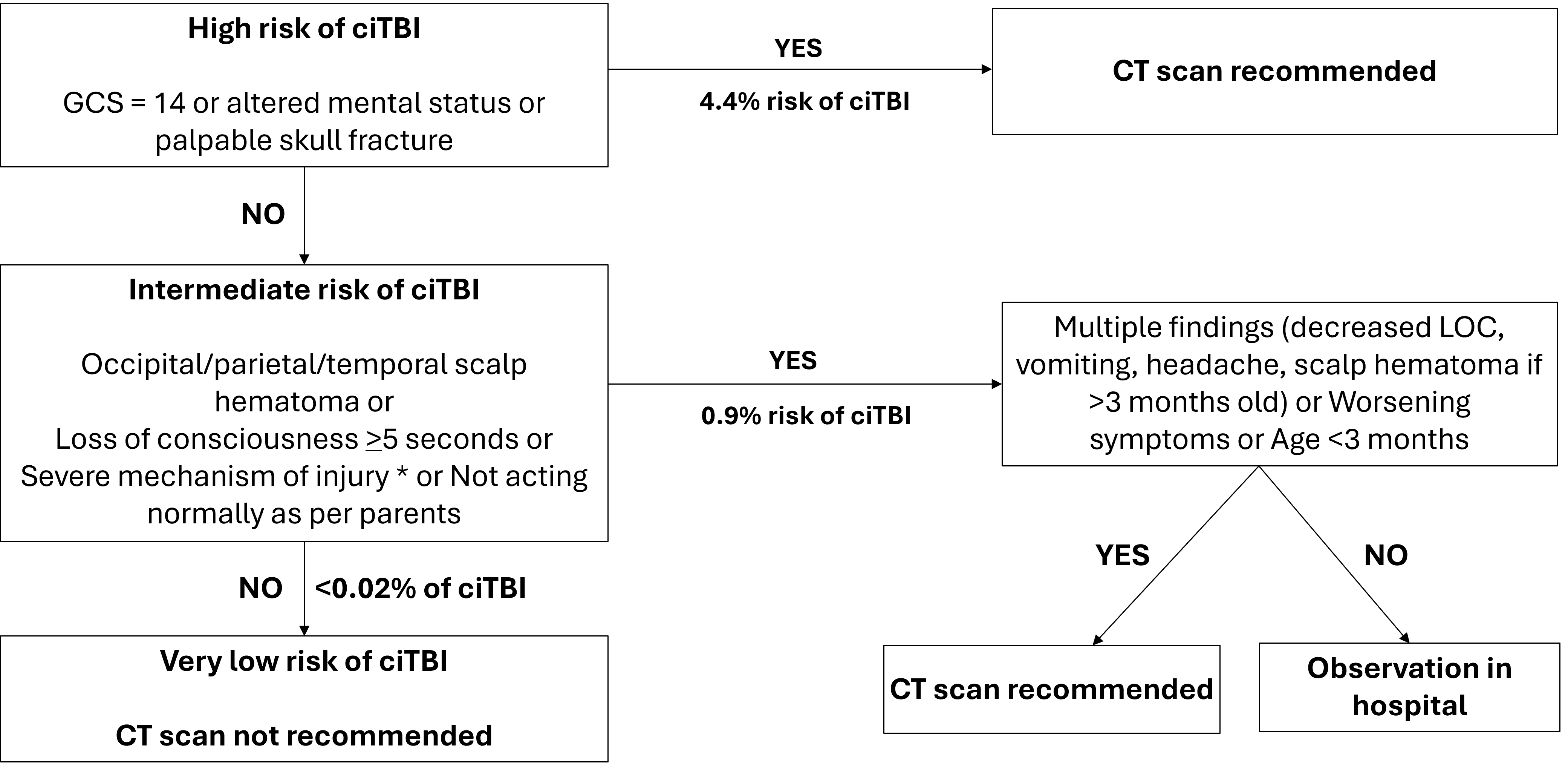
Figure 1. PECARN rules for children <2 years of age with minor head trauma (GCS 14 to 15)
Figure adapted from reference 4
Note: * refers to severe mechanism of injury: motor vehicle crash with patient ejection, death of another passenger or rollover, pedestrian or bicyclist without a helmet struck by a motorized vehicle, falls of more than 3 feet (0.9 m), or head struck by a high-impact object.
ciTBI Clinically important traumatic brain injury; CT Computed tomography; GCS Glasgow Coma Scale; LOC Level of consciousness; mo Months; sec Seconds
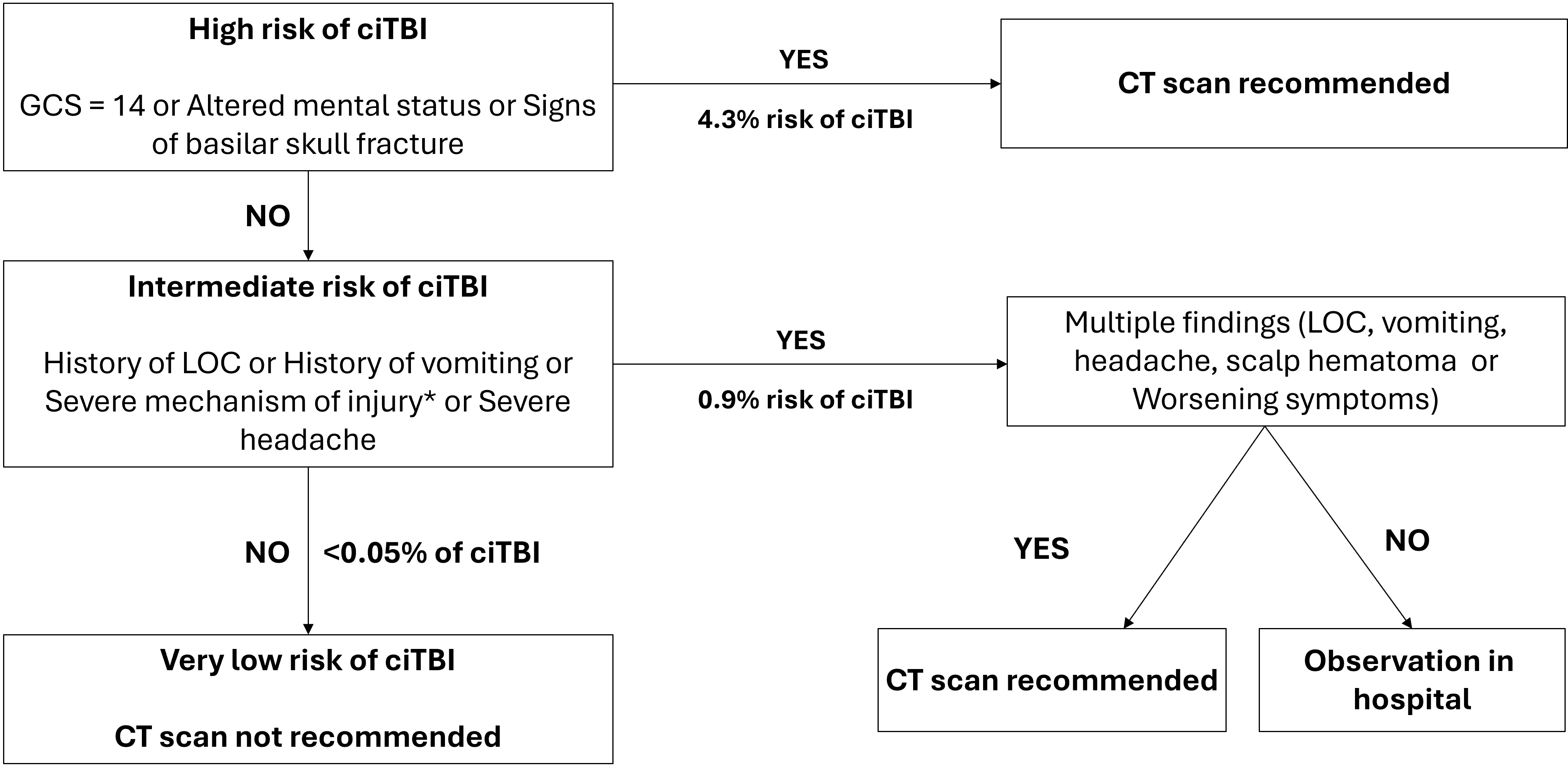
Figure 2. PECARN rules for children >2 years of age with minor head trauma (GCS 14 to 15)
Figure adapted from reference 4
Note: * refers to severe mechanism of injury: motor vehicle crash with patient ejection, death of another passenger or rollover, pedestrian or bicyclist without a helmet struck by a motorized vehicle, falls of more than 3 feet (0.9 m), or head struck by a high-impact object.
ciTBI Clinically important traumatic brain injury; CT Computed tomography; GCS Glasgow Coma Scale; LOC Level of consciousness
From the CATCH2 and CHALICE rules, other concerning signs and symptoms for consideration of a CT to rule out an ICI include:[8][21][26]
- Focal neurological deficits
- Repeated vomiting >3 times (CHALICE) or >4 times (CATCH2)
- Witnessed loss of consciousness >5 minutes
- History of amnesia >5 minutes
- Seizure with no history of epilepsy
- Tense fontanelle in child with an open fontanelle
- Bruise, swelling, or laceration of the head >5 cm if <1 year old
A known bleeding diathesis or being on a blood thinning medication is also a relative indication for imaging. Early consideration for a CT scan is appropriate for such patients.
Other imaging modalities
Indications for skull x-rays
Skull x-rays should not be performed routinely in children. Skull x-rays should only be considered in children who are <3 months of age or in children with a parietal or occipital hematoma who are not having a CT performed (Grade B, level II evidence)[27][28]. The risk of significant damage requiring neurosurgical intervention is low from isolated skull fractures[27]-[30].
When there are clinical concerns, first-line testing should focus on CT imaging when the risk of ICI is higher. There are some specific situations where a skull x-ray series may be helpful, including skeletal surveys in cases of suspected child physical maltreatment[15] to look for a radiopaque foreign body, or when CT is not easily available.
Other diagnostic imaging modalities
As an initial test, ultrasound (either point-of-care ultrasound or standard radiological exams) may be considered to detect skull fractures (Grade B, level II evidence)[31]-[33]. With appropriately skilled operators, the sensitivity and specificity of ultrasound in detecting skull fracture was approximately 90% in studies[33]. When there are clinical concerns, first-line testing should focus on CT imaging because the risk for ICI is higher. Detection of a skull fracture, if linear, would prompt a CT or extended observation in hospital until the child appears well.
In some centres with magnetic resonance imaging (MRI) availability, short sequence rapid MRI is feasible, reduces radiation, and defines intracranial injuries[34].
Management after initial assessment
Minor traumatic brain injury (GCS 14 or 15)
Asymptomatic patients without a severe mechanism of injury may be discharged home to the care of reliable parents or guardians (Grade A, level I evidence). Written instructions describing signs to watch for (e.g., worsening headache, persistent vomiting, difficulty awakening), who to contact in such a case, and when to return for medical reassessment, should be provided. Routinely waking children at night to assess neurological status is unnecessary[29].
If, after initial evaluation, there is headache or repeated vomiting, or if there was loss of consciousness at the time of trauma or a severe mechanism of injury, a period of clinical observation (4 to 6 hours) with reassessment or the option to perform CT is indicated (Grade B, level II evidence)[35]. If there is improvement in symptoms and the GCS is 15, the patient may be discharged home with parental instructions as above. When there is no improvement after the period of clinical observation, the patient should either have a CT performed or be admitted to hospital with close evaluation of neurovital signs (GCS, LOC, pupil assessment, strength and ability to move, colour, sensation and warmth, bladder function) every 2 hours. Rehydration should be provided for patients with persistent vomiting. Persistent symptoms may indicate the need for neuroimaging (CT or MRI head), if not previously performed. Positive findings on a CT scan should be discussed with a neurosurgeon, and consulting a clinician experienced in the management of head trauma may be appropriate for patients with negative CT scans but experiencing persistent symptoms.
An asymptomatic child >2 years old can be discharged home from the ED after a period of observation. In the child younger than 2 years of age, greater caution is advised (Grade D, level V evidence). Trivial head trauma in an asymptomatic, ambulatory toddler is compatible with discharge from the ED, but this may not be the case for an infant. The challenges of clinical assessment in children <2 years old and the importance of identifying THI-CM should lead clinicians to observe symptomatic patients for a longer period, with frequent reassessment [36]. When THI-CM is suspected, hospitalization is indicated and referral to child protection authorities is required.
The presence of a widened or diastatic skull fracture (>4 mm) increases the risk of developing a leptomeningeal cyst. Even without evidence of an ICI, post-discharge follow-up with neurosurgery should be arranged[37].
Moderate traumatic brain injury (GCS 9 to 13)
All patients with moderate head trauma should undergo imaging by CT scan (Grade A, level II evidence). Depending on the CT scan findings and the evolution of neurological status, admitting these patients to a paediatric intensive care unit (ICU) may be needed to provide closer monitoring. ICU monitoring is particularly needed for patients at the lower end of the GCS spectrum (i.e., GCS 9 to 10). The decision to transfer a patient with a moderate head injury to a tertiary care centre must be individualized, based on clinical judgment and local resources, and should be discussed with a paediatric intensive care or trauma team.
Severe traumatic injury (GCS <8)
Once the patient with a severe head injury has been stabilized, including intubation, a cranial CT scan should be performed (Grade A, level II evidence). Severe TBI is a complex and challenging emergency. Patients with severe head trauma are at risk of raised intracranial pressure (ICP), which can cause bradycardia, elevated blood pressure, and irregular breathing patterns (Cushing’s triad). Raised ICP may result from the mass effect of localized bleeding, as in the case of epidural and subdural hematomas, or be produced by vasogenic edema from diffuse axonal injury. In the acute setting, measures aimed at maintaining a normal ICP and cerebral perfusion pressure are appropriate[38].
Management should include: (Grade A, level I evidence)
- Continuous monitoring of vital signs and, if possible, end-tidal CO2
- Elevation of the head of the bed to 30 degrees, with the head and neck in midline position
- Mechanical ventilation to maintain normal oxygenation and ventilation
- Fluid administration + vasopressors, as required to maintain normovolemia and avoid hypotension
- Providing sedation and analgesia, particularly during procedures and transport
- Maintenance of a normal core temperature and normoglycemia
- Correction of coagulopathy
Additional measures include consideration of seizure prophylaxis, hyperosmolar therapy, and neuromuscular blockade.
Patients with severe head trauma require referral to a centre experienced in paediatric trauma with neurosurgical and paediatric critical care services (Grade A, level I evidence). During transport, continuous monitoring of neurological, respiratory, and hemodynamic status is essential, and treatment modalities for emergency management of raised ICP should be available.
Post-traumatic seizures
Most post-traumatic seizures are impact seizures that occur immediately or just after the traumatic event. Factors increasing the risk of post-traumatic seizures may include younger age,[39] severe head trauma (GCS <8), cerebral edema, subdural hematoma, and open or depressed skull fractures[40].
Seizures that occur after the initial period (30+ minutes) are of significant concern for ciTBI. These post-traumatic seizures should prompt an immediate CT scan. Post-traumatic seizures occurring in the first 30 minutes after injury with a normal neurological examination and imaging are at low risk for further complications, and patients may be discharged without seizure prophylaxis after an observation period of 4 to 6 hours (Grade B, level II evidence)[35][41].
If medications are required to treat post-traumatic seizures, acute seizure medications should be similar to those used in other contexts, such as a benzodiazepine (Ativan or midazolam), and then levetiracetam (Keppra) or phenytoin/fosphenytoin[42].
Treatment of head injury with concussion
Guidelines for management of concussion are addressed in the Canadian Paediatric Society statement titled ‘Sport-related concussion and bodychecking in children and youth’[43].
Prevention
AHT is a public health priority based on the significant impact of these injuries on children’s outcomes and health care resources. Although most injuries are minor, AHT can place a large burden on affected individuals, their families, caregivers, and society. Health care practitioners have numerous opportunities to provide age-appropriate anticipatory guidance around risk factors for head trauma in children. The CPS advocates for public policy and legislation to ensure helmet use in cycling and sporting activities, child restraint use in vehicles, regulatory legislation for recreational off-road motorized vehicles, and led the ban on baby walkers in Canada. Such measures have proven successful in reducing both the incidence and severity of traumatic head injuries in children and youth[44]. Clinicians caring for infants, children, and youth should continue to counsel families on preventing head injuries.
Key takeaways:
- Acute head trauma (AHT) is a frequent occurrence in childhood and adolescence, but few who are injured experience clinically important traumatic brain injury (ciTBI).
- A careful assessment (history and physical exam) should be conducted with every child who has AHT, especially children <2 years of age.
- Apply the Glasgow Coma Scale (GCS) to determine the severity of TBI.
- Employ the CABCD-E (catastrophic bleeding, airway, breathing, circulation, disability, and exposure) approach for acute evaluations.
- Use a clinical guideline like PECARN (Pediatric Emergency Care Applied Research Network) to evaluate whether a child requires a computed tomography (CT) scan for mild TBI.
- Skull x-rays are not recommended routinely for head injuries. Ultrasound and magnetic resonance imaging (MRI) are newer modalities that may have benefit.
- Asymptomatic patients without a severe mechanism of injury can be discharged home.
- Patients with symptoms or a severe mechanism of injury and a GCS 15 should be observed for 4 to 6 hours or have a CT performed.
- If there are any signs of deterioration, a CT should be performed.
- Moderate TBI cases should have a CT and be monitored carefully.
- For younger children, consider traumatic head injury due to child maltreatment (THI-CM).
- Early discussion or referral to the local trauma system (or both) is recommended for moderate and severe TBI.
- Severe TBI requires stabilization with the goal of maintaining normal intercranial pressure (ICP) and cerebral perfusion and pressure, and transfer to a trauma/neurosurgical/critical care centre.
- Prevention is important for decreasing the incidence of AHT.
Acknowledgements
This position statement was reviewed by the Paediatric Emergency Medicine Section Executive and Community Paediatrics and Injury Prevention Committees of the Canadian Paediatric Society. It has also been reviewed by members of the Canadian Association of Emergency Physicians (CAEP) Standards Sub-Committee.
CANADIAN PAEDIATRIC SOCIETY ACUTE CARE COMMITTEE (2020-2021)
Members: Kevin Chan MD (Chair), Kimberly Dow MD (Board Representative), Carolyn Beck MD, Karen Gripp MD (Past Member), Kristina Krmpotic MD, Marie-Pier Lirette MD (Resident Member), Kyle McKenzie MD, Evelyne D. Trottier MD
Liaisons: Laurel Chauvin-Kimoff MD (Past Chair 2012-2019), CPS Paediatric Emergency Medicine Section; Sidd Thakore MD, CPS Hospital Paediatrics Section
Principal author(s): Kevin Chan MD, Catherine A. Farrell MD, Laurel Chauvin-Kimoff MD
Funding
There is no funding to declare.
Potential Conflict of Interest
All authors: No reported conflicts of interest.
References
- Dewan MC, Mummareddy N, Wellons JC, Bondfield CM. Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurg 2016;91:479-509.e1. doi: 10.1016/j.wneu.2016.03.045
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths – United States, 2007 and 2013. MMWR Surveill Summ 2017;66(9):1-16. doi: 10.15585/mmwr.ss6609a1
- Osmond MH, Klassen TP, Wells GA, et al; Pediatric Emergency Research Canada (PERC) Head Injury Study Group. CATCH: A clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ 2010;182(4):341-8. doi: 10.1503/cmaj.091421
- Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: A prospective cohort study. Lancet 2009;374(9696):1160-70. doi: 10.1016/S0140-6736(09)61558-0
- Patricios JS, Schneider KJ, Dvorak J, et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport – Amsterdam, October 2022. Br J Sports Med 2023;57(11):695-711. doi: 10.1136/bjsports-2023-106898
- Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 2016;351(10):1014-25. doi: 10.1001/jama.2016.1203
- Sookplung P, Vavilala MS. What is new in pediatric traumatic brain injury? Curr Opin Anaesthesiol 2009;22(5):572-8. doi: 10.1097/ACO.0b013e3283303884
- Dunning J, Daly JP, Lomas JP, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child 2006;91(11):885-91. doi: 10.1136/adc.2005.083980
- Araki T, Yokota H, Morita A. Pediatric traumatic brain injury: Characteristic features, diagnosis and management. Neuro Med Chir (Toyko) 2017;57(2): 82-93.
- Glasgow Coma Scale: Do it this way (Accessed December 10, 2024).
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2(7872):81-4. doi: 10.1016/s0140-6736(74)91639-0
- University of Calgary. Pediatric Glasgow Coma Scale. Event Medicine Group. Adult and Pediatric Glasgow Coma Scale (Accessed December 10, 2024).
- Holmes JF, Palchak MJ, MacFarlane T, Kuppermann N. Performance of the Pediatric Glasgow Coma Scale in children with blunt head trauma. Acad Emerg Med 2005;12(9):814-9. doi: 10.1197/j.aem.2005.04.019
- TREKK. Peds Pac. Pediatric Multisystem Trauma Algorithm. 2024 (Accessed January 6, 2025).
- Shouldice M, Ward M, Nolan K, Cory E; Canadian Paediatric Society, Child and Youth Maltreatment Section. Medical assessment of suspected traumatic head injury due to child maltreatment (THI-CM). Paediatr Child Health 2025;30(1).
- King WJ, MacKay M, Simick A; Canadian Shaken Baby Study Group. Shaken baby syndrome in Canada: Clinical characteristics and outcomes of hospital cases. CMAJ 2003;168(2):155-9.
- Thiessen ML, Woolridge DP. Pediatric minor closed head injury. Pediatr Clin North Am 2006;53(1):1-26.v. doi: 10.1016/j.pcl.2005.09.004
- Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med 2007;357(22):2277-84. doi: 10.1056/NEJMra072149
- Choosing Wisely. CT Scans for Children with Head Injuries. (Accessed December 10, 2024).
- Parkin PC, Maguire JL. Clinically important head injuries after head trauma in children. Lancet 2009;374(9696):1127-9. doi: 10.1016/S0140-6736(09)61634-2
- Osmond MH, Klassen TP, Wells GA, et al. Validation and refinement of a clinical decision rule for the use of computed tomography in children with minor head injury in the emergency department. CMAJ 2018;190(27):E816-E822. doi: 10.1503/cmaj.170406
- Babl FE, Borland ML, Phillips N, et al. Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: A prospective cohort study. Lancet 2017;389(1087):2393-2402. doi: 10.1016/S0140-6736(17)30555-X
- Easter JS, Bakes K, Dhailwal J, Miller M, Caruso E, Haukoos JS. Comparison of PECARN, CATCH, and CHALICE rules for children with minor head injury. Ann Emerg Med 2014;64(2):145-152.e1-5. doi: 10.1016/j.annemergmed.2014.01.030
- American College of Surgeons Committee on Trauma (eds.). Advanced Trauma Life Support, Student Course Manual, Tenth Edition. Chicago, ILL: American College of Surgeons; 2018.
- MD+CALC. PECARN Pediatric Head Injury/Trauma Algorithm (Accessed December 10, 2024).
- Dunning J, Batchelor J, Starford-Smith P, et al. A meta-analysis of variables that predict significant intracranial injury in minor head trauma. Arch Dis Child 2004;89(7):653-9. doi: 10.1136/adc.2003.027722
- Gravel J, Gouin S, Chalut D, et al. Derivation and validation of a clinical decision rule to identify young children with skull fracture following isolated head trauma. CMAJ 2015;187(16):1202-08. doi: 10.1503/cmaj.150540
- Spénard S, Gouin D, Beaudin M, Gravel J. Validation of the Sainte-Justine Head Trauma Pathway for children younger than two years of age. Can J Surg 2018;61(4):283-7. doi: 10.1503/cjs.013217
- Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr 2018;172(11):e182853. doi: 10.1001/jama-pediatrics.2018.2853
- Powell EC, Atabaki SM, Wootton-Gorges S, et al. Isolated linear skull fractures in children with blunt head trauma. Pediatrics 2015;135(4):e851-7. doi: 10.1542/peds.2014-2858
- Rabiner JE, Friedman LM, Khine H, Avner JR, Tsung JW. Accuracy of point-of-care ultrasound for diagnosis of skull fractures in children. Pediatrics 2013;131(6):e1757-64. doi: 10.1542/peds.2012-3921h
- Parri N, Crosby BJ, Glass C, et al. Ability of emergency ultrasonography to detect pediatric skull fractures: A prospective observational study. J Emerg Med 2013;44(1):135-41. doi: 10.1016/j.jemermed.2012.02.038
- Parri N, Crosby BJ, Mills L, et al. Point-of-care ultrasound for the diagnosis of skull fractures in children younger than two years of age. J Pediatr 2018;196:230-6.e2. doi: 10.1016/j.jpeds.2017.12.057
- Lindberg DM, Stence NV, Grubenhoff JA, et al. Feasibility and accuracy of fast MRI versus CT for traumatic brain injury in young children. Pediatrics 2019;144(4):e20190419. doi: 10.1016/j.jpeds.2017.12.057
- Schonfeld D, Fitz BM, Nigrovic LE. Effect of the duration of emergency department observation on computed tomography use in children with minor blunt head trauma. Ann Emerg Med 2013;62(6):597-603. doi: 10.1016/j.annemergmed.2013.06.020
- Public Health Agency of Canada. Joint Statement on Traumatic Head Injury due to Child Maltreatment (THI-CM): An update to the Joint Statement on Shaken Baby Syndrome. 2020 (Accessed December 11, 2024).
- Sanford RA. Prevention of growing skull fractures. J Neurosurg Pediatr 2010;5(2):213-8. doi: 10.3171/2009.9.PEDS09180
- Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the acute medical management of severe traumatic brain injury, third edition: Update of the Brain Trauma Foundation Guidelines. Pediatr Crit Care Med 2019;20(3S Suppl 1):S1-S82. doi: 10.1097/PCC.0000000000001735
- Chiaretti A, De Benedictis R, Polidori G, Piastra M, Iannelli A, Di Rocco C. Early post-traumatic seizures in children with head injury. Childs Nerv Syst 2000;16(12):862-6. doi: 10.1007/s003810000368
- Hahn YS, Fuchs S, Flannery AM, Barthel MJ, McLone DG. Factors influencing posttraumatic seizures in children. Neurosurgery 1988;22(5):864-7.
- Badawy MK, Dayan PS, Tunik MG, et al. Prevalence of brain injuries and recurrence of seizures in children with posttraumatic seizures. Acad Emerg Med 2017;24(5):595-605. doi: 10.1111/acem.13168
- Mackenzie KC, Hahn CD, Friedman JN; Canadian Paediatric Society, Acute Care Committee. Emergency management of the paediatric patient with generalized convulsive status epilepticus. Paediatr Child Health 2021;26(1):50-66. doi: 10.1093/pch/pxaa127
- Goulet K, Beno S; Canadian Paediatric Society, Injury Prevention Committee. Sport-related concussion and bodychecking in children and youth: Evaluation, management and policy implications. Paediatr Child Health 2023;28(4):252-8. doi: 10.1093/pch/pxad007
- Yancher NL, Warda LJ, Fuselli P; Canadian Paediatric Society, Injury Prevention Committee. Child and youth injury prevention: A public health approach. November 2012.
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.