Position statement
Early detection for autism spectrum disorder in young children
Posted: Oct 24, 2019
Principal author(s)
Lonnie Zwaigenbaum, Jessica A. Brian, Angie Ip; Canadian Paediatric Society, Autism Spectrum Disorder Guidelines Task Force
Paediatr Child Health 2019 24(7):424–432. 
Abstract
Autism spectrum disorder (ASD) is a life-long neurodevelopmental disorder, characterized by impairments in social communication, repetitive, restricted patterns of behaviour, and unusual sensory sensitivities or interests. ASD significantly impacts the lives of children and their families. Currently, the estimated prevalence of ASD is 1 in 66 Canadians aged 5 to 17 years. General paediatricians, family physicians, and other health care professionals are, therefore, seeing more children with ASD in their practices. The timely diagnosis of ASD, and referral for intensive behavioural and educational interventions at the earliest age possible, may lead to better long-term outcomes by capitalizing on the brain’s neuroplasticity at younger ages. This statement provides clear, comprehensive, evidence-informed recommendations and tools to help community paediatricians and other primary care providers monitor for the earliest signs of ASD—an important step toward an accurate diagnosis and comprehensive needs assessment for intervention planning.
Keywords: Autism spectrum disorder; Developmental surveillance; Early identification; Screening
WHAT IS ASD AND HOW IS IT DEFINED IN THE DSM-5?
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with onset in early childhood that is associated with a wide range of symptoms and ability levels. As defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [1], ASD is an encompassing diagnostic category that includes two symptom domains: 1) social communication impairments, and 2) restricted, repetitive patterns of behaviours and interests. Other DSM-5 ASD diagnostic criteria are summarized in Table 1.
THE PREVALENCE, ETIOLOGY, AND RISK FACTORS FOR ASD
Prevalence
The prevalence of ASD has increased, from an estimated 1 in 1,000 children in Nova Scotia, an example cited 30 years ago [2], to a current estimate of 1 in 66 Canadians aged 5 to 17 years (1 in 42 males, and 1 in 165 females) [3]. The degree to which rising ASD prevalence is due to a true increase in cases is not yet known. Improved detection and diagnosis, and the broadening of diagnostic criteria with successive versions of the DSM, are likely contributors to changes in prevalence estimates [4]. Evidence suggests that ASD can be reliably diagnosed by 2 years of age in some children [5], though subtler cases may not present fully until later. Despite increasing awareness of early signs, the mean age of diagnosis remains 4 to 5 years of age [6]. While males are diagnosed with ASD four times more frequently than females [4][5], the sex gap may be narrowing. Recognition is growing that some girls present with more subtle signs than boys [7]–[9].
Etiology and risk factors
The etiology of ASD is not completely understood, though recent findings suggest an interplay among genetic, epigenetic, and environmental factors [10]. Strong risk factors for ASD include male sex and positive family history. Recurrence risk estimates for younger siblings of children with ASD range from 7% to 19% [11][12] versus 1.5% in the general population [4]. Recurrence varies by degree of familial relatedness. One recent Swedish study indicated a tenfold increase in relative risk when a full sibling has been diagnosed with ASD, as compared with a twofold increase when a cousin is affected [13]. Other risk factors are summarized in Table 2 [10]–[19]. The mechanisms associated with environmental exposure and ASD may include inflammation, oxidative stress, endocrine disruption and may be influenced by gene-related effects [15][16].
| Table 1. DSM-5 diagnostic criteria for ASD | |
| Domains | Criteria (with examples) |
|
1. Impairment in social interaction and communication (all three subcriteria required) |
Social and emotional reciprocity:
Impairment of nonverbal behaviours:
Failure to develop and maintain relationships:
|
|
2. Abnormal and restricted, repetitive behaviours, interests, and activities (two of four subcriteria required) |
Stereotyped speech and behaviours:
Insistence on sameness/resistance to change:
Restricted, fixated interests:
Hyper- or hypo-sensitivity to sensory input
|
| 3. Signs or symptoms must be present during early development but they may not be fully evident until later, when social demands exceed limited capacities, or they may be masked by learned strategies. | |
| 4. Symptoms interfere with everyday functioning. | |
| 5. Symptoms are not better explained by intellectual disability or global developmental delay. | |
| 6. ASD may occur with or without medical, genetic, neurodevelopmental, mental or behavioural disorders, or an intellectual or language impairment. | |
| 7. Level of severity for each of the two domains may be used to refine diagnosis: Level 1: Requiring support; Level 2: Requiring substantial support; Level 3: Requiring very substantial support. These levels may be difficult to determine at the initial time of diagnosis with very young children. | |
| Data drawn from reference [1]. ASD Autism spectrum disorder. | |
‘RED FLAGS’ AND CLINICAL PRESENTATIONS OF ASD
Overt behavioural signs of ASD are not generally present in the first 6 months of life. Prospective studies of high-risk infants suggest an emerging ASD prodrome in the latter half of the first year of life, which may include delayed motor control (e.g., persistent head lag), feeding and sleeping difficulties, and/or excessive reactivity or passivity [20][21].
Symptoms in the core domains of ASD usually emerge between 12 and 24 months. Initial presentations vary, and there is no one behavioural sign that rules an ASD diagnosis in or out. Parents’ initial concerns may include language delay, lack of response when the child’s name is said, and limited eye contact.
Other early warning signs at different stages of development are summarized in Table 3 [20]–[24]. Children with ASD may appear relatively typical with respect to early social engagement and communication, then become withdrawn or lose communication or language skills by 18 months [22]. For some children with more advanced language and cognitive skills, ASD signs are relatively subtle in the early years but become more apparent as they reach school age and begin to struggle with increasing social demands [20]–[23].
DEVELOPMENTAL SURVEILLANCE AND SCREENING
Developmental surveillance
Developmental surveillance is a flexible process whereby knowledgeable clinicians gather relevant information over time from multiple sources (including parents and by direct observation) toward the goal of identifying and addressing developmental concerns, including those related to ASD [25]. The Canadian Paediatric Society (CPS) [26] and other professional organizations [27]–[38] recommend developmental surveillance at every scheduled health visit and any time a parent or caregiver raises concerns about a child’s language or other skills development. Developmental surveillance involves integrating information obtained from inquiry around parental concerns, clinical observations and, possibly, also incorporating standardized measures (e.g., parent questionnaires) to inform clinical impressions and decision making.
Developmental screening
Developmental screening involves a brief assessment using a standardized measure to identify children at increased risk for delay or disorder. Screens vary by format (e.g., parent report versus direct assessment of the child) and scope (‘broadband’ screens cover multiple developmental domains versus those specific to a particular domain or disorder). Developmental screening also varies by target population. Universal screening targets all children regardless of level of concern, while targeted screening selects a subpopulation based on preidentified risk factors.
| Table 2. Risk factors for ASD | |
| Categories | Risk factors |
|
Genetic/familial |
Specific genetic syndromes/risk variants Male sex First-degree relative or other family history of ASD |
|
Prenatal |
Older parental age (≥35 years) Maternal obesity, diabetes, or hypertension In utero exposure to valproate, pesticide, or traffic-related air pollution Maternal infections (e.g., rubella) Close spacing of pregnancies (<12 months) |
|
Postnatal |
Low birth weight Extreme prematurity |
| Data drawn from references [10]–[19]. ASD Autism spectrum disorder. | |
| Table 3. Early warnings signs in children at risk for ASD | |
| Age (months) | Clinical presentation |
|
6–12 |
|
|
9–12 |
|
|
12–18 |
|
|
15–24 |
|
|
Any age |
|
|
Any age |
|
| Data drawn from references [20]–[24] ASD Autism spectrum disorder. | |
Table 4 summarizes the age range, time required to complete, classification properties (i.e., sensitivity and specificity), and test performance of selected broadband developmental screens [39]–[44]. Table 5 provides similar data for measures that identify children at risk for ASD. Screening implies a scoring criterion, whereby children who score above a pre-established ‘cut-point’ are classified as being at increased risk (‘screen-positive’). Clinicians may use clinical judgement when considering referral for further assessment, even when a child has a ‘screen-negative’ score. Considerations include clinical observations, parental concerns, and other suggestive factors, such as a positive family history.
CURRENT STATE OF EVIDENCE FOR ASD SCREENING
There have been several comprehensive reviews evaluating measures used as ASD screens, specifically for accuracy in particular test populations and contexts, and the evidence for (and against) their impact on age of diagnosis, access to intervention services, and long-term outcomes [21][23][45]–[47]. ASD screening evidence has also been reviewed within previous ASD assessment guidelines [28][31][35] and by the US Preventative Services Task Force (USPSTF) [48].
Several conclusions can be drawn. First, ASD screening tools have been evaluated in community contexts (e.g., paediatric primary care practices) that accurately differentiated between toddlers with and without ASD (Table 4). Second, compared with an open-ended question regarding parental concerns, some screening tools (e.g., M-CHAT and the Infant Toddler Checklist (ITC)) detected ASD earlier and more consistently [49][50]. Third, there is little evidence from clinical trials regarding how ASD screening influences diagnostic timelines and long-term outcomes. One published randomized clinical trial demonstrated younger diagnostic age by implementing the ‘Early Screen for Autism in Toddlers’ [51], although differences may have reflected collateral effects (e.g., engagement of community physicians) rather than the screen itself [52]. The lack of clinical trial evidence was cited by the USPSTF when they found insufficient evidence to ‘assess the balance of benefits and harms of screening for ASD in young children for whom no concerns of ASD have been raised by their parents or a clinician’ [48].
One recent study using simulation models found that universal screening for ASD would not result in earlier diagnosis and treatment under the Canadian health care system [53]. At time of writing, the outcomes of targeted ASD screening had not been assessed using clinical trial methodology. However, some evidence suggests that ASD screening may reduce social inequalities in accessing specialized services [54][55]. Several authors [56]–[58] have challenged whether the balance of evidence justifies excluding screening from current practice. The potential benefits of using standardized ASD symptom inventories compared with open-ended inquiry into parental concerns remains an open question.
| Table 4. General developmental assessment tools | |||||
| Parental questionnaires (For purchase except when specified) | |||||
|
Screening tool |
Age range |
Completion time |
Test performance |
Test sample |
Comments |
|
Ages and Stages Questionnaires |
1 month to 5.5 years |
10–15 minutes |
Se: 70–90% [40] Sp: 76–91% |
Children from diverse ethnic, socio-economic backgrounds |
Gross and fine motor skills, language functions, social-emotional development, adaptive skills |
|
Child Development Inventory (CDI) [41] |
15 month to 6 years |
30–40 minutes |
Se: 80–100% [41] Sp: 94–96% |
Primarily White, working class community |
300 items: 8 areas of functioning, including cognition and language |
|
Brief Early Childhood Screening Assessment (ECSA) [42] |
18–60 months |
1–5 minutes |
Se: 89% [42] Sp: 85% |
Children from primary care |
22 items: assesses emotional and behavioural development (No cost) |
|
Nipissing District Developmental Screen [43] (new name ‘Looksee Checklist’) |
1 month to 6 years |
5 minutes |
Se: 29–68% [43] Sp: 58–88% |
High-risk clinic referral group |
Commonly used in Ontario. Examines 13 developmental stages (No cost in Ontario) |
|
Parents’ Evaluation of Developmental Status (PEDS) [44] |
Birth to 8 years |
2–10 minutes |
Se: 91–97% [44] Sp: 73–86% |
Children from diverse ethnic, socio-economic backgrounds |
Expressive, receptive language, and articulation; gross motor, self-help, social-emotional, behavioural, and global-cognitive |
| Se Sensitivity; Sp Specificity | |||||
| Table 5. Commonly used, evidence-based measures of early ASD symptoms | |||||
|
Screening tool |
Age range |
Completion time |
Test performance |
Test sample |
Description |
| Questionnaires (parents, teachers) | |||||
|
Modified Checklist for Autism in Toddlers, Revised with Follow-up (M-CHAT-R/F) [30][62] |
16–30 months |
5–10 minutes |
Se: 85% [62] Sp: 91–99% PPV: 48% for ASD [20] PPV: 95% for any DD [23] |
Low-risk toddlers screened at 18- and 24-month well-child visits |
Two-stage: 20-item report with a follow-up interview (5–10 minutes). Assesses protodeclarative pointing, response to name, interest in peers, showing objects of interest to parents, imitation (No cost) |
|
8–24 months |
5–10 minutes |
Sp: 83% PPV: 71–79% NPV: 88–99% |
9- to 24-month-olds from general population |
24 items: Evaluates gestures, eye contact, facial expressions, vocalizations (No cost) |
|
|
Social Responsiveness Scale – 2nd edn. Preschool (SRS-2- Preschool) [30][63] |
2.5–4.5 years |
15–20 minutes |
Se: 75–78% Sp: 67–96% |
442 children with and without ASD |
65 items: Measures social awareness, reciprocal social communication, social anxiety, autistic traits and preoccupations |
|
Autism Spectrum Rating Scales* [64] |
2–15 years |
5–20 minutes |
Se: 92% [64] Sp: 89% PPV: 91% NPV: 89% |
2,560 children normative sample |
70 items: Assesses social and communication behaviours, self-regulation; Short-form version available (15 items, for 2- to 15-year-olds) |
| Interactive clinical tool | |||||
|
Screening tool |
Age range |
Completion time |
Psychometric properties |
Test sample |
Description |
|
Screening Tool for Autism in Two-Year- Olds* (STAT) [65] |
24–36 months |
20 minutes |
Se: 92–95% [65] Sp: 73–85% PPV: 56% NPV: 97% |
71 children with an older sibling with ASD or referred for ASD concern |
Assesses communication and social behaviours; 12 observed activities during 20-minute play sessions |
|
Rapid Interactive Screening Test for Autism in Toddlers* (RITA-T) [66] |
18–36 months |
10 minutes |
Se: 100% [66] Sp: 84% PPV: 88% |
61 toddlers from an early childhood clinic |
Differentiates toddlers with ASD and those with DD/non-ASD |
| *Training required. DD Developmental delay; NPV Negative predictive value; PPV Positive predictive value; Se Sensitivity; Sp Specificity. | |||||
RECOMMENDATIONS
The following recommendations have been developed based on optimizing early ASD diagnosis and access to interventions, objectives which are known to have positive impacts on outcome. Responsible, efficient use of health care resources, and risks related to possible misclassification (i.e., delayed identification of children with ASD, and inappropriate identification of children without ASD) have also been constant considerations.
All Canadian children should be monitored for early behavioural signs of ASD as part of general developmental surveillance.
Paediatricians, family physicians, and other health care providers should be familiar with early behavioural features of ASD (Table 3), and ask parents at every office visit whether they have any developmental concerns. A previous CPS statement recommended enhancing developmental assessment and parental education during the 18-month well-baby visit—a critical time when signs or symptoms of ASD often emerge [26].
Developmental surveillance includes collecting information on developmental concerns from parents and other care providers (e.g., grandparents and child care or toddler group staff). Relying exclusively on open-ended inquiry can under-identify children experiencing delays or disorders. Paediatric health care providers can use the Rourke Baby Record (RBR) to chart global development, physical examination data, immunization, nutrition, and other milestones [59].
Health care providers could also incorporate broadband measures to standardize information on development (Table 4) into everyday practice. However, because randomized clinical trials have not yet demonstrated that routine developmental screening for children with no preidentified risks improves outcomes, the Canadian Task Force on Preventive Healthcare (CTFPHC) has concluded that the evidence is insufficient to recommend routine screening [20][60].
It is important to recognize that the timing and clarity of early behavioural features may vary by the child’s characteristics. For example, children with milder symptoms and more advanced development tend to be diagnosed later [6]. Therefore, surveillance for any behavioural features of ASD should continue throughout childhood.
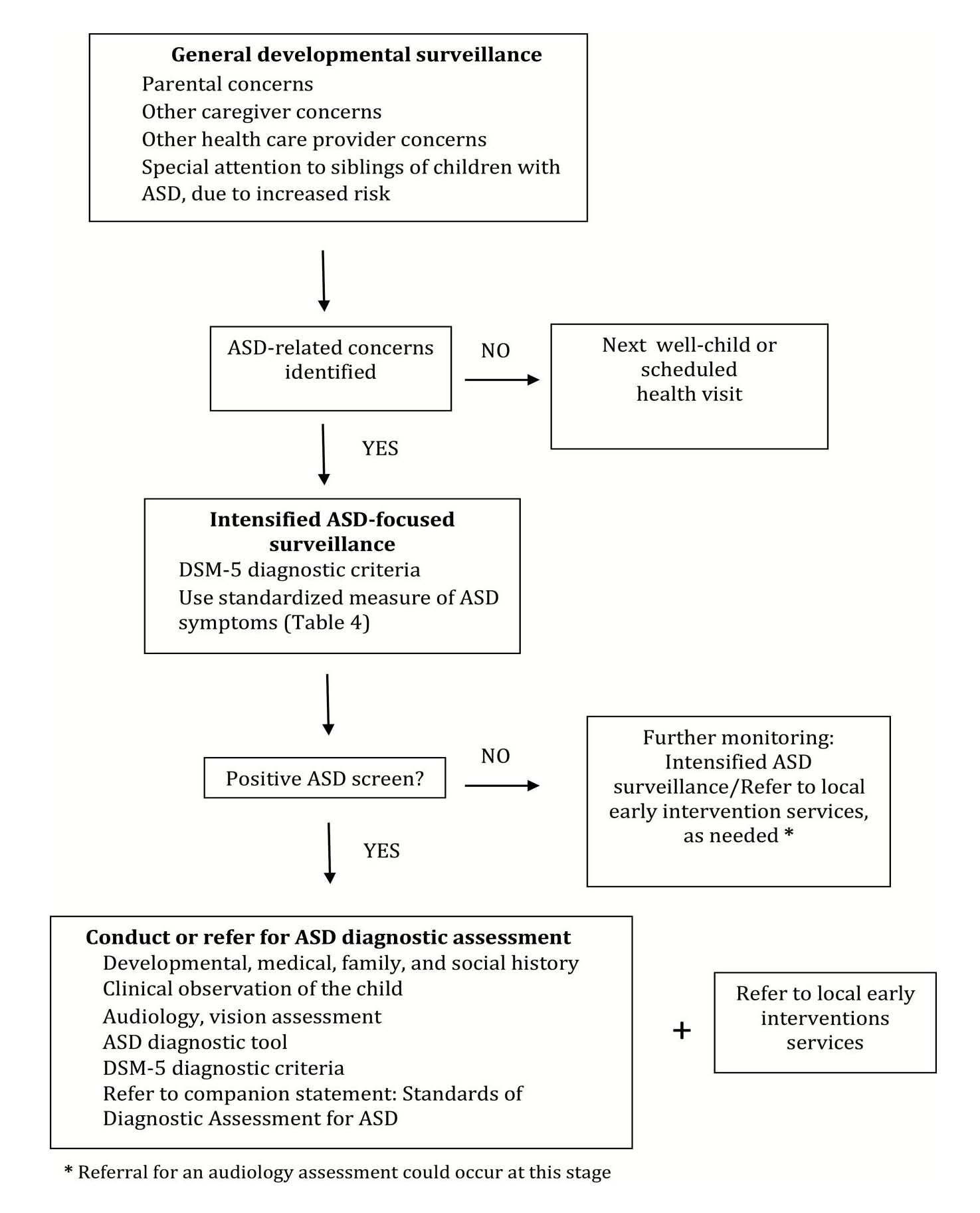
Children identified as being at increased risk for ASD should receive an early, focused evaluation to determine need for further diagnostic assessment.
When developmental surveillance indicates a possible risk for ASD, further in-depth assessment is needed. Vigilance is needed for children with known risk factors (Table 2), because the overall prevalence of ASD is higher in these children. This stage of assessment, which is more intensively ASD-focused, should include a standardized measure of ASD symptoms (Table 5) [20][23][30][47][50][61]–[65]. Either a parent questionnaire (i.e., M-CHAT-R/F or ITC) or, in communities where trained personnel are available, an interactive tool (e.g., STAT) could be used. Detailed information about symptomology complements parental and clinical observations and helps to guide next steps.
Children who meet scoring criteria according to a standardized ASD symptom measure, or whose clinical presentation indicates a high index of suspicion to their health care provider, should proceed to a diagnostic assessment, either by a community paediatrician or a specialized team (Figure 1 and the companion statement, Standards of Diagnostic Assessment for ASD, published in this issue). At-risk children should also be referred immediately for local early intervention services (e.g., infant development, speech-language therapy, occupational therapy, targeted preschool support), depending on level of need and the local service model, pending a diagnostic assessment.
To ensure that ASD diagnostic assessment is timely, accurate, and efficient, a flexible clinical pathway is proposed. This approach is tailored to the complexity of a child’s clinical presentation, a health care provider’s personal experience and clinical judgment, and the availability and scope of local health care resources (Figure 1). A paediatric health care provider may choose to make or rule out an ASD diagnosis independently, based on DSM-5 criteria and clinical judgment, or to collaborate with another professional (e.g., a paediatric (sub)specialist or child psychologist) to confirm a diagnosis (a shared care model).
When a child’s presentation is complicated by co-existing concerns, or a complex medical or psychosocial history, the community practitioner may refer the child to an expert team. Parents should be advised that their child will be referred for and receive supportive community interventions (such as speech/language or occupational therapy, or a preschool program), while waiting for a diagnostic assessment, and beyond.
- When referring a child for an ASD diagnostic assessment, include the following in the referral letter:
- Parental or health care professional reports of signs or symptoms of ASD, developmental delays or concerns, missed developmental milestones, abnormal behaviours, plus any general development or ASD screening results
- Clinical observations of signs or symptoms of ASD
- Antenatal and perinatal histories
- Developmental milestones achieved
- Any specific risk factors for ASD
- Relevant medical history and investigations
- Information from previous assessments
- Be sure to explain to parents what they can expect during any stage of the assessment process. Invite questions, and counsel with printed or online resources and contact information about local family or peer-to-peer support groups.
- Discuss concerns, fears, and feelings about the possibility of an ASD diagnosis with parents. Be sensitive to distress related to developmental concerns and potential impacts on family life. Be mindful of family vulnerability, fears for the future, and financial pressures, as they work through the assessment process.
For other clinical steps to take or initiate before a formal diagnosis is obtained, see the companion statement Standards of Diagnostic Assessment for ASD, published in this issue.
When ASD-focused surveillance does not indicate need for further diagnostic assessment, but other developmental concerns remain:
- Address these concerns directly with parents and continue ongoing developmental surveillance.
- Refer the child and parents for early, development supportive services or interventions, as appropriate.
- Revisit the need for ASD-focused monitoring if needed, and for surveillance of other developmental assessments, as concerns evolve.
How to prepare for the first office visit with a child suspected of having ASD [66][67]
- Consider scheduling a telephone call with a parent in advance of the first visit, to discuss the child’s:
- Medical and developmental history, with related family factors
- Strengths and challenges
- Sensory sensitivities that might influence behaviour within the office environment; and
- Strategies to optimize compliance during the clinical visit.
- Consider inviting both patient and parent for a ‘practice visit’ to familiarize the child with the care setting.
- Consider scheduling the child for the first (or last) appointment of the day, when there are fewer people in the waiting room, to minimize wait time.
- Schedule a longer appointment than for a typically developing child.
- Advise parent(s) to bring a couple of favourite toys or foods to offer as a distraction or reward, if needed.
- Consider re-arranging the examination room to accommodate sensory sensitivities (i.e., quiet, with dim lights).
Recommended resources:
- ASD Video Glossary (Contrasts specific developmental features in video clips of typically developing children and children with ASD): https://autismnavigator.com/asd-video-glossary/
- Autism Society: www.autismcanada.org
- Autism Speaks: www.autismspeaks.ca
- BMJ Best Practice Autism Spectrum Disorder: https://bestpractice.bmj.com/topics/en-us/379
- Canadian Paediatric Society, Caring for Kids website: Developmental milestones: (https://caringforkids.cps.ca/handouts/your_childs_development)
- CPS Screening Tools: https://cps.ca/en/tools-outils/condition-specific-screening-tools-and-rating-scales
- Centers for Disease Control and Prevention (U.S.). CDC’s developmental milestones: https://www.cdc.gov/ncbddd/actearly/milestones/index.html
- Infant Toddler Checklist (ITC): www.brookespublishing.com/resource-center/screening-and-assessment/csbs/csbs-dp/csbs-dp-itc
Funding: A systematic review funded by the Public Health Agency of Canada informed the development of the position statements.
Potential Conflicts of Interest: Dr. Zwaigenbaum reports personal fees from Roche - Independent Data Monitoring Committee (iDMC), outside the submitted work. There are no other disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
CANADIAN PAEDIATRIC SOCIETY AUTISM SPECTRUM DISORDER GUIDELINES TASK FORCE
Members: Mark Awuku MD (CPS Community Paediatrics Section), Jessica Brian PhD (co-Chair), Susan Cosgrove, Pam Green NP, Elizabeth Grier MD (College of Family Physicians of Canada), Sophia Hrycko MD (Canadian Academy of Child and Adolescent Psychiatry), Angie Ip MD, James Irvine MD, Anne Kawamura MD (CPS Developmental Paediatrics Section), Sheila Laredo MD PhD (Canadian Autism Spectrum Disorders Alliance), William Mahoney MD (CPS Mental Health Section), Patricia Parkin MD, Melanie Penner MD, Mandy Schwartz MD, Isabel Smith PhD, Lonnie Zwaigenbaum MD (co-Chair)
Principal authors: Lonnie Zwaigenbaum MD, Jessica A. Brian PhD, Angie Ip MD
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Arlington, VA: American Psychiatric Association, 2013.
- Bryson SE, Clark BS, Smith IM. First report of a Canadian epidemiological study of autistic syndromes. J Child Psychol Psychiatry 1988;29(4):433–45.
- Government of Canada. Autism Spectrum Disorder Among Children and Youth in Canada 2018: A Report of the National Autism Spectrum Disorder Surveillance System. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html (Accessed March 18, 2019).
- Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years – Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 2018;67(6):1–23.
- Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: Stability and change in clinical diagnosis and symptom presentation. J Child Psychol Psychiatry 2013;54(5):582–90.
- Daniels AM, Mandell DS. Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism 2014;18(5):583–97.
- Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. J Autism Dev Disord 2013;43(11):2584–603.
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: Setting the scene for future research. J Am Acad Child Adolesc Psychiatry 2015;54(1):11–24.
- Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2017;56(6):466–74.
- Fett-Conte AC, Bossolani-Martins AL, Rosan DBA. Etiology of autism: The complexity of risk factors in autism spectrum disorder. In: Fitzgerald, M. ed. Autism Spectrum Disorder: Recent Advances, 2015. https://www.intechopen.com/books/autism-spectrum-disorder-recent-advances/etiology-of-autism-the-complexity-of-risk-factors-in-autism-spectrum-disorder (Accessed March 18, 2019).
- Grønborg TK, Schendel DE, Parner ET. Recurrence of autism spectrum disorders in full- and half-siblings and trends over time: A population-based cohort study. JAMA Pediatr 2013;167(10):947–53.
- Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium Study. Pediatrics 2011;128(3):e488–95.
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311(17):1770–7.
- Mandy W, Lai MC. Annual research review: The role of the environment in the developmental psychopathology of autism spectrum condition. J Child Psychol Psychiatry 2016;57(3):271–92.
- Masi A, Glozier N, Dale R, Guastella AJ. The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci Bull 2017;33(2):194–204.
- Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol Autism 2017;8:13.
- Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatr Scand 2017;135(1):29–41.
- Conde-Agudelo A, Rosas-Bermudez A, Norton MH. Birth spacing and risk of autism and other neurodevelopmental disabilities: A systematic review. Pediatrics 2016;137(5):pii:e20153482.
- Emberti Gialloreti L, Mazzone L, Benvenuto A, et al. Risk and protective environmental factors associated with autism spectrum disorder: Evidence-based principles and recommendations. J Clin Med 2019;8(2):pii:E217.
- Anagnostou E, Zwaigenbaum L, Szatmari P, et al. Autism spectrum disorder: Advances in evidence-based practice. CMAJ 2014;186(7):509–19.
- Zwaigenbaum L, Bauman ML, Stone WL, et al. Early identification of autism spectrum disorder: Recommendations for practice and research. Pediatrics 2015;136(Suppl 1):S10–40.
- Thurm A, Powell EM, Neul JL, Wagner A, Zwaigenbaum L. Loss of skills and onset patterns in neurodevelopmental disorders: Understanding the neurobiological mechanisms. Autism Res 2018;11(2):212–22.
- Zwaigenbaum L, Penner M. Autism spectrum disorder: Advances in diagnosis and evaluation. BMJ 2018;361:k1674.
- Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I. The onset of autism: Patterns of symptom emergence in the first years of life. Autism Res 2008;1(6):320–8.
- Dworkin PH. Detection of behavioral, developmental, and psychosocial problems in pediatric primary care practice. Curr Opin Pediatr 1993;5(5):531–6.
- Williams R, Clinton J; Canadian Paediatric Society, Early Years Task Force. Getting it right at 18 months: In support of an enhanced well-baby visit. Paediatr Child Health 2011;16(10):647–54.
- Dua K. Standards and Guidelines for the Assessment and Diagnosis of Young Children with Autism Spectrum Disorder in British Columbia: An Evidence-based Report Prepared for the British Columbia Ministry of Health Planning, March 2003. http://www.phsa.ca/Documents/asd_standards_0318.pdf (Accessed March 18, 2019).
- The Miriam Foundation. Screening, Assessment and Diagnosis of Autism Spectrum Disorders in Young Children: Canadian Best Practice Guidelines. https://www.miriamfoundation.ca/DATA/TEXTEDOC/Handbook-english-webFINAL.pdf (Accessed March 18, 2019).
- New Zealand Ministy of Health. New Zealand Autism Spectrum Disorder Guideline, 2nd edn, 2016. Wellington, NZ. https://www.health.govt.nz/publication/new-zealand-autism-spectrum-disorder-guideline (Accessed March 18, 2019).
- Missouri Autism Guidelines Initiative. Autism Spectrum Disorders: Missouri Best Practice Guidelines for Screening, Diagnosis, and Assessment; A 2010 Consensus Publication. https://www.autismguidelines.dmh.mo.gov/ (Accessed March 18, 2019).
- National Institute for Health and Care Excellence. Autism Spectrum Disorder in Under 19s: Recognition, Referral and Diagnosis (Clinical guideline 128) London, UK: NICE, 2011 (updated December 2017). https://www.nice.org.uk/guidance/cg128 (Accessed March 18, 2019).
- University of Connecticut School of Medicine and Dentistry, Connecticut Guidelines for a Clinical Diagnosis of Autism Spectrum Disorder, 2013. Articles – Patient Care, 45. http://digitalcommons.uconn.edu/pcare_articles/45 (Accessed March 18, 2019).
- New York State Department Of Health Bureau Of Early Intervention. Best Practice Protocol for Early Screening of Young Children for Autism Spectrum Disorders (ASDs) by Pediatric Primary Care Providers, July 2013. https://www.health.ny.gov/community/infants_children/early_intervention/autism/docs/best_practice_protocol.pdf (Accessed March 25, 2019).
- Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J, State M; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2014;53(2):237–57.
- Johnson CP, Myers SM. Council on Children with Disabilities. Identification and evaluation of children with autism spectrum disorders. Reaffirmed 134(5):E1520. Pediatrics 2007;120(5):1183–1215.
- Scottish Intercollegiate Guidelines Network (SIGN). Assessment, Diagnosis and Interventions for Autism Spectrum Disorders. Edinburgh, Scotland: 2016: http://www.sign.ac.uk (Accessed March 18, 2019).
- Whitehouse AJO, Evans K, Eapen V, Wray J. A National Guideline for the Assessment and Diagnosis of Autism Spectrum Disorders in Australia. Brisbane, Australia: Autism Cooperative Research Centre (CRC), 2018.
- HAS Haute Autorité de Santé. Autism spectrum disorder: Warning signs, Detection, Diagnosis and Assessment in Children and Adolescents. Best practice guidelines, February 2018. www.has-sante.fr/jcms/c_468812/en/autism-spectrum-disorder-warning-signs-detection-diagnosis-and-assessment-in-children-and-adolescents?cid=fc_1249601&user Lang=en&portal=r_1482172&userLang=en (Accessed August 2, 2019).
- Moodie S, Daneri P, Goldhagen S, Halle T, Green K, LaMonte L. Early Childhood Developmental Screening and a Compendium of Measures for Children Ages Birth To Five. Early childhood developmental screening: A compendium of measures for children ages birth to five (OPRE Report 201411). Washington, DC: Office of Planning, Research and Evaluation, Administration for Children and Families, U.S. Department of Health and Human Services, 2014.
- Squires J, Twombly E, Bricker D, Potter L. ASQ-3 User’s Guide. 3rd edn. Baltimore, MD: Paul H. Brookes Publishing, 1999.
- Doig KB, Macias MM, Saylor CF, Craver JR, Ingram PE. The child development inventory: A developmental outcome measure for follow-up of the high-risk infant. J Pediatr 1999;135(3):358–62.
- Fallucco EM, Wysocki T, James L, Kozikowski C, Williams A, Gleason MM. The brief early childhood screening assessment: Preliminary validity in pediatric primary care. J Dev Behav Pediatr 2017;38(2):89–98.
- Cairney J, Clinton J, Veldhuizen S, et al. Evaluation of the revised Nipissing District Developmental Screening (NDDS) tool for use in general population samples of infants and children. BMC Pediatr 2016;16:42.
- Glascoe FP. Parents’ Evaluation of Developmental Status (PEDS). Nashville, TN: Ellsworth & Vandermeer Press, 2006.
- Hampton J, Strand PS. A review of level 2 parent-report instruments used to screen children aged 1.5-5 for autism: A meta-analytic update. J Autism Dev Disord 2015;45(8):2519–30.
- Stewart LA, Lee LC. Screening for autism spectrum disorder in low- and middleincome countries: A systematic review. Autism 2017;21(5):527–39.
- Towle PO, Patrick PA. Autism spectrum disorder screening instruments for very young children: A systematic review. Autism Res Treat 2016;2016:4624829.
- U.S. Preventive Services Task Force. Final Recommendation Statement: Autism Spectrum Disorder in Young Children; Screening. September 2017. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/autism-spectrum-disorder-in-young-children-screening (Accessed March 18, 2019).
- Robins DL. Screening for autism spectrum disorders in primary care settings. Autism 2008;12(5):537–56.
- Wetherby AM, Brosnan-Maddox S, Peace V, Newton L. Validation of the infanttoddler checklist as a broadband screener for autism spectrum disorders from 9 to 24 months of age. Autism 2008;12(5):487–511.
- Swinkels SH, Dietz C, van Daalen E, Kerkhof IH, van Engeland H, Buitelaar JK. Screening for autistic spectrum in children aged 14 to 15 months. I: The development of the Early Screening of Autistic Traits questionnaire (ESAT). J Autism Dev Disord 2006;36(6):723–32.
- Oosterling IJ, Wensing M, Swinkels SH, et al. Advancing early detection of autism spectrum disorder by applying an integrated two-stage screening approach. J Child Psychol Psychiatry 2010;51(3):250–8.
- Yuen T, Carter MT, Szatmari P, Ungar WJ. Cost-effectiveness of universal or highrisk screening compared to surveillance monitoring in autism spectrum disorder. J Autism Dev Disord 2018;48(9):2968–79.
- Durkin MS, Elsabbagh M, Barbaro J, et al. Autism screening and diagnosis in low resource settings: Challenges and opportunities to enhance research and services worldwide. Autism Res 2015;8(5):473–6.
- Herlihy LE, Brooks B, Dumont-Mathieu T, et al. Standardized screening facilitates timely diagnosis of autism spectrum disorders in a diverse sample of low-risk toddlers. J Dev Behav Pediatr 2014;35(2):85–92.
- Coury DL. Babies, bathwater, and screening for autism spectrum disorder: Comments on the USPSTF recommendations for autism spectrum disorder screening. J Dev Behav Pediatr 2015;36(9):661–3.
- Pierce K, Courchesne E, Bacon E. To screen or not to screen universally for autism is not the question: Why the task force got it wrong. J Pediatr 2016;176:182–94.
- Mandell D, Mandy W. Should all young children be screened for autism spectrum disorder? Autism 2015;19(8):895–6.
- Rourke L, Leduc D. The Rourke Baby Record. https://cps.ca/en/tools-outils/rourke-baby-record (Accessed March 18, 2019).
- Tonelli M, Parkin P, Brauer P, et al. Canadian Task Force on Preventive Health Care. Recommendations on screening for developmental delay. CMAJ 2016;188(8):579–87.
- Robins DL, Casagrande K, Barton M, Chen CM, Dumont-Mathieu T, Fein D. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics 2014;133(1):37–45.
- Constantino JN, Gruber CP. Social responsiveness scale – Second Edition (SRS-2). Los Angeles, CA: Western Psychological Services, 2012.
- Goldstein S, Naglieri JA. Autism Spectrum Rating Scales (ASRS): Product Overview. Multi-Health Systems, 2009, 2010. https://www.pearsonclinical.co.uk/Psychology/ChildMentalHealth/ChildAutisticSpectrumDisorders/AutismSpectrumRatingScales/Resources/ASRS-Product-Overview.pdf (Accessed March 25, 2019).
- Stone WL, McMahon CR, Henderson LM. Use of the screening tool for autism in two-year-olds (STAT) for children under 24 months: An exploratory study. Autism 2008;12(5):557–73.
- Choueiri R, Wagner S. A new interactive screening test for autism spectrum disorders in toddlers. J Pediatr 2015;167(2):460–6.
- Autism Canada. Autism Physician Handbook: Canadian edition. https://autismcanada.org/resources/physician-handbook/ (Accessed March 18, 2019).
- HANDS in Autism. Toolkit for Medical Professionals: Tips and Supports for Working with Individuals with Autism Spectrum Disorders. Indianapolis, IN: Christian Sarkine Autism Treatment Center, Riley Child & Adolescent Psychiatry Clinic, Indiana University School of Medicine – Department of Psychiatry, 2008. https://handsinautism.iupui.edu/pdf/Toolkit-Intro.pdf (Accessed March 18, 2019).
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.