Position statement
Best practices in pain assessment and management for children
Posted: Nov 4, 2022
Principal author(s)
Evelyne D. Trottier MD, Samina Ali MD, Marie-Joëlle Doré-Bergeron MD, Laurel Chauvin-Kimoff MD; Canadian Paediatric Society, Acute Care Committee, Hospital Paediatrics Section, Paediatric Emergency Medicine Section
Paediatr Child Health 2022 27(7):429–437. 
Abstract
Pain assessment and management are essential components of paediatric care. Developmentally appropriate pain assessment is an important first step in optimizing pain management. Self-reported pain should be prioritized. Alternatively, developmentally appropriate behavioural tools should be used. Acute pain management and prevention guidelines and strategies that combine physical, psychological, and pharmacological approaches should be accessible in all health care settings. Chronic pain is best managed using combined treatment modalities and counselling, with the primary goal of attaining functional improvement. The planning and implementation of pain management strategies for children should always be personalized and family-centred.
Keywords: Analgesia; Assessment; Paediatrics; Pain; Treatment
Background
Historically, children’s pain has been under-recognized [1] and under-treated [2], and while progress in both assessment and treatment has been made in recent years, a knowledge-to-practice gap remains. Pain is under-evaluated in many settings [3], with the youngest children [4] and individuals who are cognitively impaired being the most negatively impacted [5][6]. Multiple reasons for suboptimal treatment of pain are reported, including the difficulty of assessing pain in children [7], lack of time and resources, and educational, cultural, and legal factors [8].
Suboptimal pain management can have negative consequences in the short and longer term [8], including progression of acute discomfort and distress, increased fear and pain during subsequent medical visits, the development of chronic pain, and future avoidance of medical care [9]. In contrast, adequate pain management is associated with faster recovery and reduced health care resource utilization (10). Treating pain does not delay diagnosis or decision-making [11][12], and often improves the health care provider’s (HCP’s) ability to evaluate and treat children [9].
This position statement focuses on timely, developmentally appropriate pain assessment and management strategies for children in a family-centred context. It complements the Canadian Paediatric Society (CPS) statement on procedural pain published in 2019 [13].
Pain assessment
Assessment is an essential step for optimal pain management [14]. Appropriate assessment includes pain location, quality, duration, and intensity, and also requires use of a developmentally appropriate tool (Table 1) and repeated assessment(s) post-intervention. Particularly for chronic pain, a child’s psychosocial context, with impacts on daily life (e.g., family, sleep, play, school) should also be assessed [15].
Self-reported pain scales
Pain is a subjective experience that can be modulated by emotions, developmental factors, culture, current context, and previous pain experiences [14][15]. Whenever possible, children’s pain should be evaluated through self-report rather than by proxy. Pain scales should be employed as directed, using consistent, standardized scale anchors to facilitate comparison.
Among the more than 60 readily available measurement tools, the most recommended scales include: the verbal Numeric Rating Scale-11 (NRS-11), the Faces Pain Scale-Revised (FPS-R) (Figure 1), and the Color Analogue Scale (CAS) [16]. These scales work best for acute presenting pain but have also been used in the post-operative setting. No single self-report scale has been strongly endorsed for children <6 years old or for the assessment of chronic pain [16][17].
The NRS-11 scale ranks pain severity from 0 (no pain) to 10 (worst pain possible or imaginable). The NRS-11 can be used for children ≥6 years old with acute pain [16][18]. It can also be used, though with less supporting evidence, for post-operative and chronic pain. The main advantage of the NRS-11 is that it is verbally administered.
The FPS-R uses six facial images, ranging from 0 (no pain) to 10 (worst pain). Children sometimes prefer FPS-R to the NRS-11 and Visual Analogue Scale (VAS) [14][15]. The FPS-R can be used in children aged 4 to 7 years old [14][15] to evaluate acute and, with less evidence, post-operative pain [16]. FPS-R is not recommended to evaluate chronic pain.
Figure 1. Faces Pain Scale-Revised
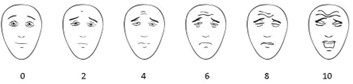
Note: Consult the International Association for the Study of Pain (IASP) website for details.
The CAS invites children to move a slider along the scale’s length to indicate how much pain they have (from ‘‘no pain’’ to ‘‘most pain’’), with a 0 to 10 scale printed on the back. CAS is used in children ≥8 years old, mainly to evaluate acute and, with less evidence, post-operative pain. Evidence to support the use of CAS for chronic pain assessment has been inconclusive [16].
Chronic pain assessment requires an evaluation of its multiple dimensions, with critical focus on the impacts of pain on a child’s global functioning (e.g., school attendance, activities, socializing, sleep) [19]. Validated and readily available chronic pain assessment tools include the Bath Adolescent Pain Questionnaire (BAPQ), the Patient Reported Outcome Measurement Information System’s (PROMIS’s) Pediatric Pain Interference Scale (PPIS), and the Pediatric Pain Questionnaire (PPQ) [15]. For more information, see the Holland Bloorview Kids Rehabilitation Hospital’s Toolbox for Children with Disabilities [20]. Emotional functioning measures for anxiety, depressive symptoms, and pain catastrophizing, (i.e., when an individual magnifies, dwells on, is pessimistic about or dreads pain), along with sleep measures, should also be employed to complete the evaluation [19].
Observational pain measures
For pre-verbal and non-verbal children, evaluating behavioural signs such as facial expression, cry, irritability, poor feeding, sleep disturbance, and inactivity, can help decode a child’s discomfort level [21]. Changing vital signs may be correlated with pain in infants, but are not reliable indicators in older children [4][21][22]. Notably, an absence of change in vital signs does not indicate lack of pain in children.
When self-report of pain is not possible (e.g., due to young age, cognitive impairment, mechanical ventilation), a behavioural pain scale or checklist should be used [23]. For children with cognitive disability, never assume that a self-report cannot be obtained [4]. If extent of intellectual disability truly precludes a child’s ability to self-report pain, an observational scale should be used in tandem with a parent or caregiver's assessment. While self-reporting tools can be used by a caregiver or HCP to obtain a proxy assessment, caution is advised, particularly when an evaluator is not familiar with the child [23].
Out of the more than 55 observational pain instruments currently available [4][24][25], only a small number are recommended [25]. For infants, the Neonatal Infant Pain Scale (NIPS), and the Face, Legs, Activity, Cry, Consolability (FLACC) scale are recommended most often [4], though one recent systematic review has also suggested that the EValuation ENfant DOuLeur (EVENDOL), COMFORT, and Neonatal Facial Coding System (NFCS) scales carry lower risk for bias than other tools [25]. Alternative scales to consider for infant pain are the Échelle de Douleur et d’Inconfort du Nouveau-né (EDIN), to assess prolonged pain in preterm infants; the Neonatal Pain, Agitation, and Sedation Scale (N-PASS) for acute and prolonged pain; and the Premature Infant Pain Profile (PIPP) for procedural pain in preterm and term neonates [25].
For toddlers and older children, the FLACC or the CHEOPS (Children’s Hospital of Eastern Ontario Pain Scale) scales are also considered reliable and validated tools [23]. However, some research has suggested that the EVENDOL, COMFORT, and NFCS scales are better choices due to lower risk for bias [25].
The NIPS is a 0 to 7 point scale comprising physiological, behavioural, and contextual indicators that was developed to assess procedural pain in newborns and older infants [24][26].
The NFCS measures acute, procedural, or prolonged pain by assessing facial cues (25). For more information on pain assessment and management in neonates, see this joint statement from the CPS and American Academy of Pediatrics (AAP) [27].
The FLACC 0 to 10 scale is recommended for evaluating post-operative pain in children 2 months to 7 years old. The FLACC is not optimal for evaluating procedural pain or for children who show fewer physical or vocal manifestations of pain [28][29]. Further, developmentally appropriate positioning (e.g., swaddling limits activity evaluation) can interfere with FLACC assessment [7][29]. A revised scale (r-FLACC) includes measures for assessing cognitively impaired or non-verbal children 4 to 19 years old [4][29][30] (Figure 2).
Figure 2. FLACC and r-FLACC scales
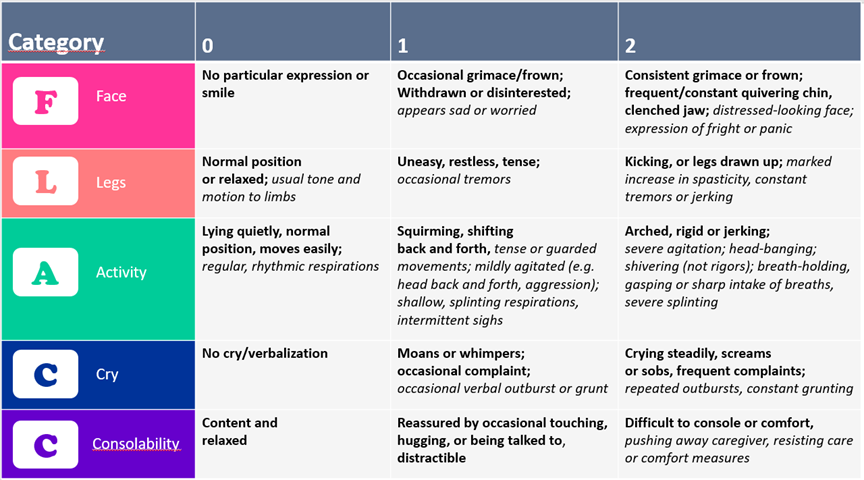
Adapted from Malviya 2006 [30] combining the original FLACC (text in bold) and the addition of the r-FLACC (text in italic)
The EVENDOL is a validated 15-point scale developed for children 0 to 7 years old in the emergency department and can be used for presenting, prolonged, and procedural pain [25][33]. It is particularly useful for children who show fewer physical or vocal manifestations of pain and is validated for use in prehospital settings [34].
The CHEOPS is a 14-point scale that measures post-operative pain. The main challenge with use is that a score of 4 to 6 represents no pain, which appears counterintuitive. Moreover, some studies have suggested that the CHEOPs scale is not appropriate for evaluating procedural pain [28]. However, the MBPS (Modified Behavioral Pain Scale), a modified version of the CHEOPS, has been validated for evaluating immunization-related pain in children 2 to 22 months old, though is not recommended for other procedures or age groups [31][32].
Instructions:
- Assess at rest (R): observe the child from a distance, before performing any examination or procedure, at rest, ensuring the best possible conditions of safety and comfort, for example with his/her parents, when he/she is playing.
- During examination or mobilization (M): assess pain during examination or mobilization or palpation of the painful area by nurse or by doctor.
- Reassess pain regularly after analgesic administration: wait 30 to 45 minutes if analgesic is administered by oral or rectal route, 5 to 10 minutes if administered by IV route. Note whether the child is at rest (R) or mobilized (M).

From reference 31. Reproduced with permission
The COMFORT scale takes time to administer, but has been validated and adapted for use in children of all ages who are undergoing mechanical ventilation [23][25][26].
A valid behavioural scale for children experiencing chronic pain has not yet been identified [23].
Timing of assessment
Evaluating a child’s pain at first encounter is recommended, with regular reassessments during care. Re-evaluating pain following every intervention and during every procedure is essential for assessing therapeutic efficacy. To optimize comparison, the same scale(s) should be used each time (Table 1) [21]. The dual goals of pain measurement are to relieve children’s pain and assess their response to treatment.
Table 1. Recommended pain scales
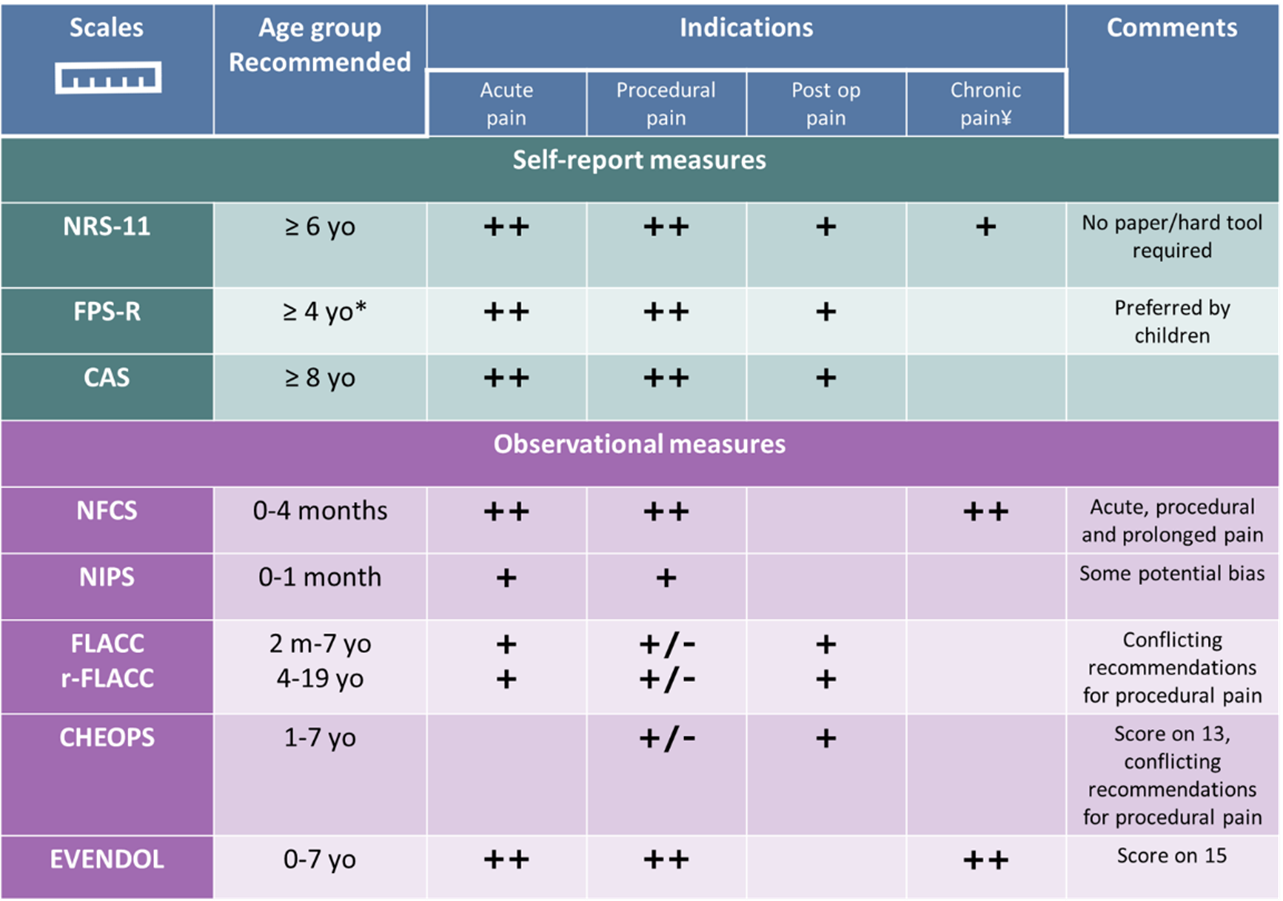
*Recommended for ≥7 yo by some authors [16]
Supported by evidence: ++: strong (least bias, high relevance, strongly supported by evidence); +: good (some bias, moderate support in recommendations, recommended in some reviews, but less/more weakly by others; +/-: limited (limited support/conflicting recommendations between studies). Based on the reviews from references [4][16][23][25][29].
†See also validated tools for chronic pain.
CAS: Color Analog Scale, CHEOPS: Children’s Hospital of Eastern Ontario Pain Scale, EVENDOL: Evaluation Enfant, DOuLeur, FLACC: Face, Legs, Activity, Cry, Consolability, FPS-R: Faces Pain Scale-Revised, NFCS: Neonatal Facial Coding System, NIPS: Neonatal Infant Pain Scale, NRS-11: Numeric Rating Scale-11, r-FLACC: Face, Legs, Activity, Cry, Consolability (revised) for children with cognitive impairment, yo: years old
Acute pain management
Pain is the most common reason to seek medical attention in all acute care settings in Canada [21]. There is strong evidence that pain can be treated early without affecting diagnostic accuracy [4][11][12]. In fact, pain relief often makes examination and testing easier, thereby facilitating diagnosis. A growing body of evidence supports many different effective pain management strategies. General pain treatment includes pharmacological but also, concomitantly and equally importantly, physical and psychological therapies. Treatment of pain in the health care setting must include preventing and managing pain associated with procedures. Adjunctively with pain prevention see the ‘3 P’ approach described in a previous CPS statement [13] and the acute pain toolkit from Children’s Healthcare Canada (CHC) [35].
Pain management guidelines and protocols
Early pain assessment and management should be an essential component of any health care encounter. Advocating for nurse- or paramedic-initiated medical directives can empower these health professionals to address children’s pain needs in a timely fashion. For example, standing orders to administer analgesia or apply topical anesthetic before venipuncture in acute care settings facilitates early pain relief and may improve a child’s experience. Access to tools for distraction while awaiting a medical consultation or treatment can further improve the overall medical experience.
First-line medications
Combined with physical and psychological strategies, over-the-counter (OTC) analgesics can be used as monotherapy for mild to moderate (1–3 to 4–6/10) pain or as co-therapy for moderate to severe pain (4–6 to 7–10/10). OTC analgesics include acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen (Table 2).
Table 2. Dosing of pain medications for children and youth
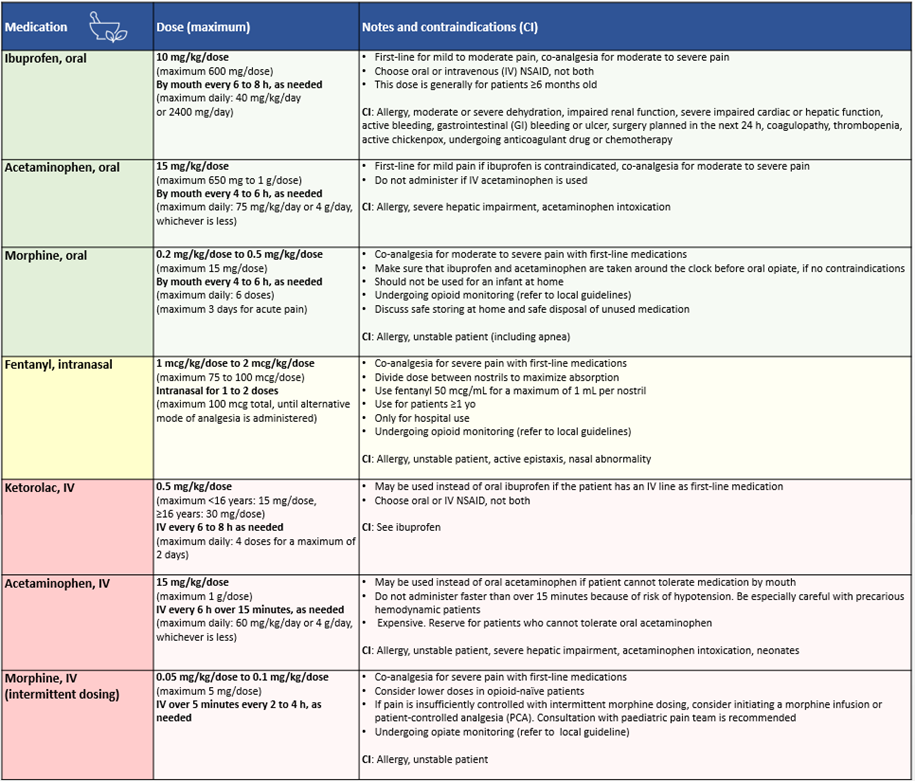
NSAID: non-steroidal anti-inflammatory drug; yo: years old
Ibuprofen is more effective than acetaminophen for the treatment of children’s pain [36], particularly for acute pain, musculoskeletal trauma, headache, and post-dental extraction [21][37][38] and has a comparable safety profile [36][39]. Moreover, ibuprofen is comparable to oral morphine for sprains, simple fractures, and following minor orthopedic procedures and tonsillectomy, with less risk for adverse events [40][43]. The combination of acetaminophen and ibuprofen to manage dental extraction or tonsillectomy pain is superior compared with acetaminophen used alone [21].
Opioid analgesia
Patients presenting with acute moderate to severe pain unlikely to be resolved by physical and psychological strategies and first-line analgesics should be offered pharmacological interventions on an escalating basis. Opioids should be used judiciously, with appropriate dosing assessment to avoid adverse events, and careful monitoring (Table 2). Co-therapy with OTC analgesics should be ongoing due to opioid-sparing effects and to reduce adverse events [21][39][44][45]. Codeine should never be used in children under 18 years old, as per Health Canada directives [46][47].
In the context of the international opioid crisis, concerns regarding substance misuse and dependency following even short-term therapeutic use in youth have emerged [48][50]. However, they should not discourage appropriate opioid use for children and youth presenting with acute severe pain or who are not responding to first-line treatments. In medical settings, opioid administration should be as rapid and painless as possible. When required at discharge [3], prescriptions of no more than five to ten doses are advised for a 2 to 3 day period [50]. This prescription must be accompanied by instructions for follow-up if pain relief is inadequate, safe storage at home, and safe disposal of unused medication (i.e., returning it to a pharmacy) [48][51]. See Solutions for Kids in Pain resources [51].
Intranasal (IN) fentanyl is an effective analgesic for treating acute moderate to severe pain in children and causes minimal distress at administration [52][53]. IN fentanyl allows for faster and less painful administration than the IV or intramuscular (IM) routes, and has a faster onset of action than oral delivery. Also safe and effective in the prehospital setting [54], IN fentanyl is the opioid of first-line for moderate to severe pain in Canadian paediatric EDs [55]. It has also been evaluated as a safe and effective initial treatment for painful vaso-occlusive episodes (VOE) in sickle cell disease (SCD), reducing time to first parenteral opioid and the number of IV insertions [56][58].
IV opioids are often used preferentially in acute medical settings, once vascular access has been established [21], with IV morphine being the most common agent [59]. IV and IN opioids must be monitored appropriately and titrated to effect, always considering severity of the patient’s pain and whether they are opioid-naive.
Analgesic adjuncts and alternatives
Numerous agents and routes of administration are being explored in the acute care setting. Recently, sub-dissociative dosing of IN ketamine has been shown to have comparable efficacy to IN fentanyl for limb injuries, but with increased risk for adverse effects [60][62]. IV ketorolac appears to have opioid-sparing effects, but its role as monotherapy remains unclear [21]. Other emerging options in Canada include IV acetaminophen. One retrospective study has reported promising results for IV acetaminophen in reducing pain in children with SCD presenting with VOE [63], but further study is required (Table 2).
Chronic pain
Chronic pain (defined as persistent or recurrent for >3 months) is a common problem that can significantly impact social, economic, and health outcomes for children, youth, and families. Pain can be secondary to underlying chronic conditions such as SCD or inflammatory bowel disease, or part of a primary pain disorder, as with irritable bowel syndrome or complex regional pain syndrome [64][66]. Headaches, abdominal, and musculoskeletal pain are the most prevalent forms of paediatric chronic pain [67]. Chronic pain prevalence rates in children range between 11% and 38% [67], are generally higher in girls, and increase with age (except for abdominal pain, which tends to present in younger children). Associated factors include lower socioeconomic status, anxiety, depression, and low self-esteem [67]. Frequency of chronic pain in children rises when there is a parental history of chronic pain [68].
About 5% of children and youth living with chronic pain are functionally disabled by it (i.e., missing out on school and social activities) [64][69]. Pain catastrophizing [70], can further influence pain-related behaviours, such as avoiding activity or physical hypervigilance.
Neuropathic pain can be secondary to several different conditions, such as trauma, surgery, cancer (or its treatment), autoimmune disorders (e.g., Guillain-Barré syndrome), or rare diseases (e.g., mitochondrial disorders) [71][72]. Few studies on managing neuropathic pain in children have been published, and current guidance is based largely on experience with adults.
Chronic pain management
Chronic pain is best managed by combining treatment modalities, including psychological, physical, occupational, and pharmacological therapies. Approaches should be individualized, with symptom severity, level of function, co-morbidities (including anxiety or depression), and the child’s and family’s ability to actively engage in therapy being prime considerations. The primary initial focus of treatment is functional improvement rather that pain reduction per se [70]. In cases of severe functional disability, or when symptom intensity has not improved despite multimodal outpatient treatment, referral to an interdisciplinary chronic pain treatment program is needed [69][70].
Psychological therapies include psychoeducation, physiological self-regulation training (e.g., biofeedback, hypnosis), cognitive skills training, and behavioural exposure therapy. Involving parents and caregivers in pain and coping strategies education is key to success [73]. The goal of physical therapy is to assess and treat secondary musculoskeletal impairments and use evidence-based chronic pain strategies, such as gradual exposure to movement, pacing, and graded motor imaging to increase tolerance of physical activity and optimize participation. Occupational therapy (OT) helps promote sensory rehabilitation to decrease sensitivity to stimuli that are perceived as painful. OT helps the child to get back to activities of daily living and self-care [70]. Pharmacological therapy may include antidepressants (e.g., amitriptyline) or antiepileptics (e.g., gabapentin, pregabalin). There is no quality evidence for the pharmacological treatment of chronic non-cancer pain in children [74]. For more on chronic pain management strategies, see the chronic pain toolkit from Children’s Healthcare Canada [35].
Empowering patients, parents, and caregivers
Patient- and family-centred care are essential to pain management, and parents, children, and youth all have active roles to play in pain management and health care planning [75]. At home, a child’s acute pain should be self-assessed whenever possible, and when a proxy is needed, parents or caregivers should be taught to assess a child’s pain regularly using developmentally appropriate tools [23][76]. To facilitate both acute and chronic pain management at home, involving parents and caregivers in clinical encounters informs and empowers them to provide optimal pain management later on. Psychological strategies, such as distraction, can be equally useful on the home front, and physical care strategies, such as appropriate wound dressing or encouraging physical activity, are important, teachable ‘take home’ skills. Adequate analgesics should be prescribed, with detailed guidance on how best to prevent pain and risk for adverse events. Clear, written instructions on medication dose, frequency, and duration of use must be provided [35][77].
Recommendations
- Education on the use of developmentally appropriate pain assessment tools is an essential first step to providing optimal pain management in paediatrics.
- Whenever possible, children’s pain should be self-reported. When self-reporting is not possible or appropriate, an appropriate assessment tool should be used.
- In medical settings, pain management can be improved through:
- Mandatory pain assessment at the first encounter with children and youth,
- Timely reassessment (and documentation) during and after procedures, as appropriate, and following every clinical intervention,
- Integrating pain assessment and management steps into treatment algorithms, electronic medical records, hospital guidelines, and regional or provincial/territorial guidelines.
- Pain prevention and management guidelines must include psychological, physical, and pharmacological strategies for acute and chronic pain. Health care directives and practice guidelines should combine strategies and approaches in the health care setting and for use at home.
- When managing chronic pain in paediatrics, relief of symptoms and improved function are the primary goals.
- Parents and caregivers should be counselled and trained in appropriate pain assessment and management and provided with clear instructions for therapy and medication use at home.
Acknowledgements
We wish to acknowledge Drs. Krista Baerg and Naveen Poonai for reviewing this statement and providing thoughtful input. The statement was also reviewed by the Community Paediatrics and Drug Therapy and Hazardous Substances Committees of the Canadian Paediatric Society.
CANADIAN PAEDIATRIC SOCIETY ACUTE CARE COMMITTEE (2020-2021)
Members: Carolyn Beck MD, Kevin Chan MD (Chair), Kimberly Dow MD (Board Representative), Karen Gripp MD, Kristina Krmpotic MD, Marie-Pier Lirette MD (Resident Member), Evelyne D. Trottier MD
Liaisons: Laurel Chauvin-Kimoff MD (Past Chair 2012-2019), CPS Paediatric Emergency Medicine Section; Sidd Thakore MD, CPS Hospital Paediatrics Section
CANADIAN PAEDIATRIC SOCIETY HOSPITAL PAEDIATRICS SECTION (2020-2021)
Executive members: Melanie Buba MD (President Elect), Marie-Joëlle Doré-Bergeron MD (Past President), Jessica Foulds MD (Member at Large), Smita Roychoudhury (Resident Liaison), Sepideh Taheri MD (Member at Large), Sidd Thakore MD (President), Kevin Weingarten MD (Secretary-Treasurer)
CANADIAN PAEDIATRIC SOCIETY PAEDIATRIC EMERGENCY MEDICINE SECTION (2020-2021)
Executive members: Kevin Chan MD (Past President), Carolyn Cashin MD (Secretary-Treasurer), Laurel Chauvin-Kimoff MD (President), Dayae Jeong MD (Resident Member), April Kam MD (Member at Large), Michelle Long MD (Vice President), Andrea Robb MD (Member at Large)
References
- Bulloch B, Garcia-Filion P, Notracia D, Bryson M, McConahay T. Reliability of the color analog scale: Repeatability of scores in traumatic and nontraumatic injuries. Acad Emerg Med 2009:16(5):465-9.
- Birnie KA, Chambers CT, Fernandez CV, et al. Hospitalized children continue to report un-dertreated and preventable pain. Pain Res Manag 2014;19(4):198-204.
- Paquin H, Trottier ED, Robitaille N, Pastore Y, Bergeron MJ, Bailey B. Oral morphine proto-col evaluation for the treatment of vaso-occlusive crisis in paediatric sickle cell patients. Paediatr Child Health 2019;24(1):e45-e50.
- Drendel AL, Kelly BT, Ali S. Pain assessment for children: Overcoming challenges and opti-mizing care. Pediatr Emerg Care 2011;27(8):773-81.
- Malviya S, Voepel-Lewis T, Merkel S, Tait AR. Difficult pain assessment and lack of clinician knowledge are ongoing barriers to effective pain management in children with cognitive impair-ment. Acute Pain 2005;7(1):27-32.
- Hauer J, Houtrow AJ; Section on Hospice and Palliative Medicine, Council on Children with Disabilities. Pain assessment and treatment in children with significant impairment of the central nervous system. Pediatrics 2017;139(6):e20171002.
- Crellin DJ, Harrison D, Santamaria N, Huque H, Babl FE. The psychometric properties of the FLACC scale used to assess procedural pain. J Pain 2018;19(8):862-72.
- Brennan F, Carr DB, Cousins M. Pain management: A fundamental human right. Anesth Analg 2007;105(1):205-21.
- Ali S, McGrath T, Drendel AL. An evidence-based approach to minimizing acute procedural pain in the emergency department and beyond. Pediatr Emerg Care 2016;32(1):36-42.
- Stinson JN, McGrath P. No pain – all gain: Advocating for improved paediatric pain man-agement. Paediatr Child Health 2007;12(2):93-4.
- Bailey B, Bergeron S, Gravel J, Bussières JF, Bensoussan A. Efficacy and impact of intrave-nous morphine before surgical consultation in children with right lower quadrant pain suggestive of appendicitis: A randomized controlled trial. Ann Emerg Med 2007;50(4):371-8.
- Poonai N, Paskar D, Konrad SL, et al. Opioid analgesia for acute abdominal pain in chil-dren: A systematic review and meta-analysis. Acad Emerg Med 2014;21(11):1183-92.
- Trottier ED, Bergeron MJ, Chauvin-Kimoff L, Baerg K, Ali S; Canadian Paediatric Society, Acute Care Committee, Hospital Paediatrics Section, Community Paediatrics Section, Paediatric Emergency Care Section. Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures. Paediatr Child Health 2019;24(8):509-21
- Huguet A, Stinson JN, McGrath PJ. Measurement of self-reported pain intensity in children and adolescents. J Psychosom Res 2010;68(4):329-36.
- Manworren RC, Stinson J. Pediatric pain measurement, assessment, and evaluation. Semin Pediatr Neurol 2016;23(3):189-200.
- Birnie KA, Hundert AS, Lalloo C, Nguyen C, Stinson JN. Recommendations for selection of self-report pain intensity measures in children and adolescents: A systematic review and quality assessment of measurement properties. Pain 2019;160(1):5-18.
- Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics 2010;126(5):e1168-98.
- Bailey B, Daoust R, Trottier ED, Dauphin-Pierre S, Gravel J. Validation and properties of the verbal numeric scale in children with acute pain. Pain 2010;149(2):216-21.
- Palermo TM. Assessment of chronic pain in children: Current status and emerging topics. Pain Res Manag 2009;14(1):21-6.
- Kingsnorth S, Townley A, Provvidenza C ,et al. Chronic Pain Assessment Toolbox for Children with Disabilities: Section 3.0; Chronic pain assessment tools. To-ronto, Ont.: Holland Bloorview Kids Rehabilitation Hospital, Version 2, 2018 (Accessed November 25, 2021).
- Bailey B, Trottier ED. Managing pediatric pain in the emergency department. Paediatr Drugs 2016;18(4):287-301.
- Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in chil-dren in the emergency department. Lancet 2016;387(10013):83-92.
- von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain 2007;127(1-2):140-50.
- Cong X, McGrath JM, Cusson RM, Zhang D. Pain assessment and measurement in neo-nates: An updated review. Adv Neonatal Care 2013;13(6):379-95.
- Giordano V, Edobor J, Deindl P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: A systematic review. JAMA Pediatr 2019;173(12):1186-97.
- Beltramini A, Milojevic K, Pateron D. Pain assessment in newborns, infants, and children. Pediatr Ann 2017;46(10):e387-e395.
- Barrington KJ, Batton DG, Finley GA, Wallman C; Canadian Paediatric Society, Fetus and Newborn Committee. Joint statement with the AAP: Prevention and management of pain in the neonate: An update. Pediatrics 2006;118(5):2231-41
- Crellin D, Sullivan TP, Babl FE, O’Sullivan R, Hutchinson A. Analysis of the validation of ex-isting behavioral pain and distress scales for use in the procedural setting. Paediatr Anaesth 2007; 17(8):720-33.
- Crellin DJ, Harrison D, Santamaria N, Babl FE. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: Is it reliable, valid, and feasi-ble for use? Pain 2015;156(11):2132-51.
- Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impair-ment. Pediatr Anesthesia 2006;16 (3): 258-65.
- Taddio A, Hogan ML, Moyer P, et al. Evaluation of the reliability, validity and practicality of 3 measures of acute pain in infants undergoing immunization injections. Vaccine 2011;29(7):1390-4.
- Crellin DJ, Babl FE, Santamaria N, Harrison D. A systematic review of the psychometric properties of the Modified Behavioral Pain Scale (MBPS). J Pediatr Nurs 2018;40:14-26.
- Fournier-Charrière E, Tourniaire B, Carbajal R, et al. EVENDOL, a new behavioral pain scale for children ages 0 to 7 years in the emergency department: Design and validation. Pain 2012;153(8):1573-82
- Beltramini A, Galinski M, Chabernaud JL, et al. Pain assessment in children younger than 8 years in out-of-hospital emergency medicine: Reliability and validity of EVENDOL score. Pediatr Emerg Care 2019;35(2):125-31.
- Knowledge Exchange Network (KEN)/Children’s Healthcare Canada (CHS). Pediatric Pain, toolkits and resources (Accessed November 8, 2021).
- Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: A meta-analysis and qualitative review. Ann Pharmacother 2010;44(3):489-506.
- Le May S, Ali S, Khadra C, et al. Pain management of pediatric musculoskeletal injury in the emergency department: A systematic review. Pain Res Manag 2016;2016:4809394.
- Tan F, Braithwaite I, McKinlay C, Dalziel SR. Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than 2 years: A systematic review and meta-analysis. JAMA Netw Open 2020;3(10):e2022398.
- Hartling L, Ali S, Dryden DM, et al. How safe are common analgesics for the treatment of acute pain for children? A systematic review. Pain Res Manag 2016;2016:5346819.
- Poonai N, Bhullar G, Lin K, et al. Oral administration of morphine versus ibuprofen to man-age postfracture pain in children: A randomized trial. CMAJ 2014;186(18):1358-63.
- Poonai N, Datoo N, Ali S, et al. Oral morphine versus ibuprofen administered at home for postoperative orthopedic pain in children: A randomized controlled trial. CMAJ 2017;189(40):E1252-E1258.
- Le May S, Ali S, Plint AC, et al. Oral analgesics utilization for children with musculoskeletal injury (OUCH Trial): An RCT. Pediatrics 2017:140(5):e20170186.
- Kelly LE, Sommer DD, Ramakrishna J, et al. Morphine or ibuprofen for post-tonsillectomy analgesia: A randomized trial. Pediatrics 2015;135(2):307-13.
- Ferland CE, Vega E, Ingelmo PM. Acute pain management in children: Challenges and re-cent improvements. Curr Opin Anaesthesiol 2018;31(3):327-32.
- American College of Emergency Physicians. Optimizing the Treatment of Acute Pain in the Emergency Department. 2017 (Accessed October 27, 2021).
- Government of Canada. Health Canada’s review recommends codeine only be used in patients aged 12 and over. 2013 (Accessed October 27, 2021).
- Government of Canada. Recalls and Safety Alerts: Non-prescription Pain Relief Products Containing Codeine Are Not Recommended for Use in People Under 18 Years of Age. 2020 (Accessed October 27, 2021).
- Ahrari M, Ali S, Hartling L, et al. Nonmedical opioid use after short-term therapeutic expo-sure in children: A systematic review. Pediatrics 2021;148(6):e2021051927.
- Institute for Safe Medication Practices Canada. Safer Decisions Save Lives: Key Opioid Prescribing Messages for Community Practitioners. ISMP Canada Safety Bulletin 2016;16 (8) (Accessed October 27, 2021).
- Health Quality Ontario. Evidence to Improve Care: Opioid Prescribing for Acute Pain. 2018 (Accessed October 27, 2021).
- Reiter E, Ali; Solutions for Kids in Pain. So You have been Prescribed an opioid? (Accessed November 8, 2021).
- Mudd S. Intranasal fentanyl for pain management in children: A systematic review of the literature. J Pediatr Health Care 2011;25(5):316-22.
- Murphy A, O’Sullivan R, Wakai A, et al. Intranasal fentanyl for the management of acute pain in children. Cochrane Database Syst Rev 2014(10):CD009942.
- Murphy AP, Hughes M, McCoy S, Crispino G, Wakai A, O’Sullivan R. Intranasal fentanyl for the prehospital management of acute pain in children. Eur J Emerg Med 2017;24(6):450-54.
- Fowler M, Ali S, Gouin S, et al. Knowledge, attitudes and practices of Canadian pediatric emergency physicians regarding short-term opioid use: A descriptive, cross-sectional survey. CMAJ Open 2020;8(1):E148-E155.
- Kavanagh PL, Sprinz PG, Wolfgang TL, et al. Improving the management of vaso-occlusive episodes in the pediatric emergency department. Pediatrics 2015;136(4):e1016-25.
- Akinsola B, Hagbom R, Zmitrovich A, et al. Impact of intranasal fentanyl in nurse initiated protocols for sickle cell vaso-occlusive pain episodes in a pediatric emergency department. Am J Hematol 2018. Doi:10.1002/ajh.25144.
- Paquin H, Trottier ED, Pastore Y, Robitaille N, Doré Bergeron MJ, Bailey B. Evaluation of a clinical protocol using intranasal fentanyl for treatment of vaso-occlusive crisis in sickle cell pa-tients in the emergency department. Paediatr Child Health 2020;25(5):293-99.
- Poonai N, Zhu R. Analgesia for children in acute pain in the post-codeine era. Curr Pediatr Rev 2018;14(1):34-40.
- Graudins A, Meek R, Egerton-Warburon D, Oakley E, Seith R. The PICHFORK (Pain in Chil-dren Fentanyl or Ketamine) trial: A randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med 2015;65(3):248-254.e1.
- Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of intranasal ketamine vs fentanyl on pain reduction for extremity injuries in children: The PRIME randomized clinical trial. JAMA Pediatr 2019;173(2):140-46.
- Reynolds SL, Bryant KK, Studnek JR, et al. Randomized controlled feasibility trial of intrana-sal ketamine compared to intranasal fentanyl for analgesia in children with suspected extremity fractures. Acad Emerg Med 2017;24(12):1430-40.
- Baichoo P, Asuncion A, El-Chaar G. Intravenous acetaminophen for the management of pain during vaso-occlusive crises in pediatric patients. P T 2019;44(1):5-8.
- Friedrichsdorf SJ, Giordano J, Dakoji KD, Warmuth A, Daughtry C, Schulz CA. Chronic pain in children and adolescents: Diagnosis and treatment of primary pain disorders in head, abdomen, muscles and joints. Children (Basel) 2016;3(4):42.
- Schechter NL. Functional pain: Time for a new name. JAMA Pediatr 2014;168(8): 693-4.
- Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain 2015;156(6):1003-1007.
- King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011;152(12):2729-38.
- McKillop HN, Banez GA. A broad consideration of risk factors in pediatric chronic pain: Where to go from here? Children (Basel)2016;3(4):38.
- Miró J, Huguet A, Nieto R. Predictive factors of chronic pediatric pain and disability: A Del-phi poll. J Pain 2007;8(10):774-92.
- Harrison LE, Pate JW, Richardson PA, Ickmans K, Wicksell RK, Simons LE. Best-evidence for the rehabilitation of chronic pain. Part 1: Pediatric pain. J Clin Med 2019;8(9):1267.
- Walco GA, Dworkin RH, Krane EJ, LeBel AA, Treede RD. Neuropathic pain in children: Spe-cial considerations. Mayo Clin Proc 2010;85(3 Suppl):S33-41.
- Verriotis MA, Walker SM. Neuropathic pain in children. In: McGrath PJ, Stevens BJ, Walker SM, Zempsky WT, eds. Oxford Textbook of Paediatric Pain. Oxford, U.K.: Oxford University Press, 2021.
- Simons LE, Basch MC. State of the art in biobehavioral approaches to the management of chronic pain in childhood. Pain Manag 2016;6(1):49-61.
- Eccleston C, Fisher E, Cooper TE, et al. Pharmacological interventions for chronic pain in children: An overview of systematic reviews. Pain 2019;160(8):1698-1707.
- Whiston C, Ali S, Wright B, et al. Is caregiver refusal of analgesics a barrier to pediatric emergency pain management? A cross-sectional study in two Canadian centres. CJEM 2018;20(6):892-902.
- Drendel A. Spotlight Pain Scale. 2014 (Accessed November 25, 2021)
- Institute for Safe Meditation Practices Canada. 5 Questions to Ask about Your Medica-tions. 2021 (Accessed October 27, 2021).
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.


