Position statement
Diagnosis and management of congenital syphilis – Avoiding missed opportunities
Posted: Mar 28, 2024
Principal author(s)
Sergio Fanella MD, Ari Bitnun MD, Michelle Barton MD, Laura Sauve MD; Canadian Paediatric Society, Infectious Diseases and Immunization Committee
Abstract
Congenital syphilis can result in pregnancy loss and substantial morbidity in newborns. The current epidemic of congenital syphilis in Canada (especially Western Canada) is a preventable public health emergency. Rates indicate a lack of control of syphilis within the community and insufficient public health resources to prevent spread that predate the COVID-19 pandemic. Risk factors include lack of prenatal care, methamphetamine and other substance use, and unstable housing. The cornerstone of prevention is identification, treatment, and follow-up during pregnancy, including of partners. Clinicians caring for newborns need to consider aspects of maternal treatment, reinfection risk, the results of paired maternal and infant syphilis serology, and infant clinical assessment. A complete risk assessment will guide effective management and follow-up of infants exposed in utero to syphilis.
Keywords: Penicillin; Pregnancy; Serology; Syphilis
Background
Mother-to-child transmission of Treponema pallidum subspecies pallidum (syphilis) can occur in utero or intrapartum/postpartum through contact with an infectious maternal lesion of the mucous membranes or skin. Transmission rates are highest in primary and secondary maternal syphilis (>70%) compared with early latent (~40%) and late latent (<10%) stages[1]. Maternal infection is often asymptomatic or associated with non-specific symptoms such as fever, rash, or genital ulcers, which are often mistaken for other conditions. Spontaneous abortion, fetal demise, and late-term stillbirth occur in about one-third of untreated early pregnancy infections. An overview of acquired syphilis, including stages, clinical findings, and diagnostic aspects, can be found elsewhere[2].
Congenital syphilis (CS) is entirely preventable. However, despite decades of awareness and standardized testing and treatment recommendations, cases continue to occur for a variety of reasons, including inadequate maternal treatment or suboptimal screening due to lack of prenatal care, failure to provide timely treatment despite positive serological results, reinfection after treatment or, in some cases, the failure to treat sexual partners.
Canadian context
There has been a significant resurgence of syphilis in Canada over the past two decades. From 2011 to 2020, rates of infectious syphilis increased 385%, with the largest relative increases from 2016 to 2020 in Western Canada (Alberta 484%, Manitoba 537%, Saskatchewan 891%)[3]. Associated risk factors for syphilis can include: injection drug use; crystal methamphetamine or other substance use; the sale or purchase of sex; experiencing homelessness; inconsistent condom use; having multiple partners; and having had other sexually transmitted and bloodborne infections (STBBIs) recently diagnosed[1]. Rates of CS increased substantially from 2018 through 2020, from 4.6 cases per 100,000 live births to 13.4 in 2020[3]. Regions from Western Canada were disproportionally affected[4].
Understanding syphilis diagnostics and interpretation in pregnancy
The potential for asymptomatic syphilis infection and its nonspecific or subtle maternal disease manifestations make serology the cornerstone of diagnosis. At a minimum, syphilis serology is recommended at the time of the first prenatal visit, with recommendations for repeat testing at 28 to 32 weeks and at delivery in areas with outbreaks or for individuals with ongoing risk of infection[2]. Repeat testing should also be performed in the context of clinical suspicion of maternal reinfection, a new maternal STI at any point during pregnancy (e.g., gonorrhea, chlamydia), in case of a stillbirth after 20 weeks gestation, or in accordance with provincial/territorial guidelines. Newborn infants ideally should not be discharged from hospital until results of maternal syphilis testing are known and appropriate steps for management are arranged.
Two types of serological tests for syphilis are available: treponemal-specific tests (TTs) and nontreponemal tests (NTTs). Both must be used to adequately manage patients, including infants with suspected CS. TTs are qualitative but more sensitive and specific than NTTs. NTTs are semiquantitative and therefore can provide a measure of disease stage and activity.
Treponemal testing detects antibodies specific for Treponema species, including T. pallidum subspecies pallidum. TTs are generally positive for life following infection and cannot be used to monitor treatment response. Examples of TTs include chemiluminescent microparticle immunoassay (CMIA), T. pallidum particle agglutination (TP-PA), and fluorescent treponemal antibody absorption (FTA-ABS).
Nontreponemal testing utilizes cardiolipin, cholesterol, and lecithin, an antigen shared in the membranes of mammalian cells as well as in T. pallidum. NTTs include a rapid plasma reagin (RPR) test or a venereal disease research laboratory (VDRL) test. Both can be run off blood, but RPR is the preferred test for blood while VDRL is the preferred test of cerebral spinal fluid (CSF). These NTTs are nonspecific and can be falsely positive with a variety of conditions including collagen-vascular diseases, pregnancy, malignancy, certain viral infections (e.g., Epstein-Barr virus), or other spirochete-related infections such as Lyme disease.
NTT titers rise during the early stages of infection (primary and secondary syphilis) and then decline gradually over time, whether treatment is administered or not. NTTs fall more rapidly with successful treatment and following NTT titers over time is important. A change in NTT titer of fourfold or greater difference is considered significant (two dilutions; e.g., from 1:128 to 1:32). A twofold or one dilution change is generally not considered significant due to the subjective nature of test result interpretation. In the context of early-stage infection, a fourfold or greater drop in RPR titer, generally within a year following treatment, is required to confirm that treatment has been effective. In a subset of individuals who have been adequately treated, the NTT titers may remain detectable for a prolonged or indefinite period, usually at a low level, a scenario referred to as a “serofast” state.
A reactive TT result may also precede a reactive NTT result in early primary infection. Therefore, repeat testing in 4 to 6 weeks is warranted if the TT is reactive and the NTT test is non-reactive in certain situations. Late latent and tertiary syphilis are often characterized by a reactive TT with a non-reactive NTT (because NTT can decline over time without treatment). Infection with endemic treponema (i.e., yaws, pinta, bejel) can cause reactive TT and NTT results. These chronic treponemal non-venereal infections are typically acquired in developing regions of the world and lack the late cardiovascular and neurologic complications that can occur in syphilis.
Direct detection of T. pallidum can be used to confirm active maternal disease. Testing may be performed on ulcers or other mucocutaneous lesions or secretions, CSF, amniotic fluid, and in the setting of stillbirth (e.g., placenta, umbilical cord). Darkfield microscopy is rarely available for routine clinical use, even in major Canadian centres. Immunohistochemistry or polymerase chain reaction (PCR) testing are now preferred as direct detection methods and to confirm maternal disease.
Two approaches to syphilis screening in pregnancy are used in Canada and elsewhere, following either a traditional or a reverse algorithm, with most using the latter[5]. The traditional approach uses an initial NTT which, if reactive, is followed by one or more TTs. The reverse algorithm performs a TT first, followed by an NTT with or without a second TT. Advantages of the second approach include the ability to detect antibodies earlier in primary syphilis and elimination of the biological false positives associated with NTTs.
Treatment in pregnancy
The preferred treatment for infectious syphilis in pregnancy consists of long-acting benzathine penicillin G, administered intramuscularly (IM), with the number of doses determined by the maternal stage of syphilis. (Crystalline penicillin G intravenously for 10 days is the treatment for maternal neurosyphilis.) Primary, secondary, and early latent syphilis are treated with one dose of benzathine penicillin G, with some experts and jurisdictions recommending an additional dose. Three doses are recommended for late latent syphilis or in situations where the stage of disease is uncertain[6]. No antibiotic apart from benzathine penicillin (or crystalline penicillin G to treat maternal neurosyphilis) is considered an acceptable substitute for prevention of CS at the present time.
The minimum standard of maternal treatment that is considered adequate includes an appropriate number of doses of IM benzathine penicillin G for maternal disease stage (or IV penicillin G if neurosyphilis for 10 days), with course completed >4 weeks before delivery. For treatment to be deemed successful, a fourfold or greater drop in NTT titer must be documented.
Treatment of later stage maternal syphilis may begin with already low NTT titers, and a fourfold drop may not be achievable before delivery. An adequate number of treatment doses completed ≥4 weeks before delivery can help guide later management decisions in such cases, and can be discussed with an infectious diseases (ID) specialist, as needed.
Assessing infants born to mothers with reactive syphilis serology during pregnancy
1. Appropriate maternal treatment
The first step in caring for infants born to mothers with reactive serology is to review maternal records and determine whether adequate treatment was received and whether treatment was effectiive (Figure 1). Formal documentation of treatment should be sought through local public health authorities regarding specific dates of therapy and results from serial NTT titers during post-treatment follow-up. On history, reported injections may be medications other than penicillin (e.g., IM ceftriaxone for empiric gonorrhea infections). To assess risk for reinfection, clinicians should include a careful history of the mother’s sexual partners, including the most recent sexual encounter with each, whether partners were treated, and when they were treated relative to the mother’s treatment. Timely discussions with maternal care providers may provide additional information regarding maternal risks and other treatment aspects.
Figure 1. Determining adequacy of treatment of syphilis in pregnancy
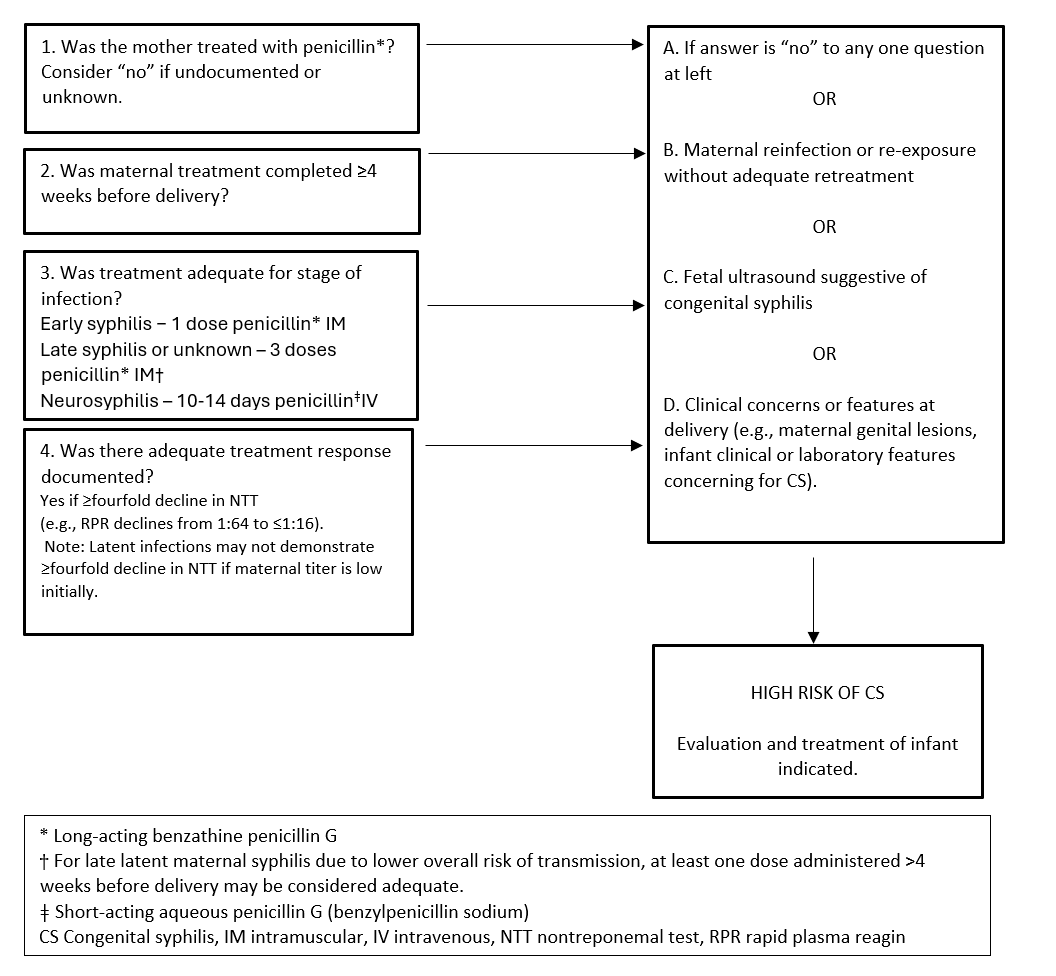
2. Infant physical examination
All newborns, including those born to mothers with reactive syphilis NTT/TT, should have a physical examination looking for signs of CS. The range of clinical presentations of CS are summarized in Table 1. At birth, 50% to 90% of infants will be asymptomatic, and findings may not be present until weeks to months later. Several references provide a more thorough overview of the clinical spectrum of CS[7]-[9]. For infants with CS diagnosed in infancy but outside the neonatal period, clinical manifestations can be similar, with a significant number also having long bone or CSF abnormalities[10].
Table 1. Clinical features and presentations of early congenital syphilis (CS)
| System | Feature |
|---|---|
| Growth | Low birth weight; failure to thrive* |
| General | Fever, pallor, jaundice, nonimmune hydrops, generalized lymphadenopathy, severe sepsis syndrome |
| Head and neck | Rhinitis, chorioretinitis, cataracts, uveitis, keratitis |
| Skin | Maculopapular*, desquamating*, or vesiculobullous lesions |
| Cardiorespiratory | Myocarditis, congestive heart failure, respiratory distress, pneumonia |
| Gastrointestinal | Hepatosplenomegaly*, necrotizing funisitis, pancreatitis, transaminitis |
| Central nervous system | Cranial neuropathies, meningitis, seizures, hearing loss |
| Musculoskeletal | Dactylitis, periostitis* leading to pseudoparalysis |
| Renal | Proteinuria, hematuria, or nephrotic syndrome |
| Other | May mimic other infectious diseases syndromes or congenital infections and non-infectious conditions (e.g., juvenile myelomonocytic leukemia) |
*Common findings
3. Comparing maternal and infant NTT titers
Paired maternal-infant sera samples should be ordered in the immediate post-partum period to allow comparison of NTT titers. Infant samples should be from venipuncture, not from the umbilical cord, because umbilical cord blood can be contaminated by maternal blood and yield a false-positive result. Serological testing should be expedited whenever possible to allow for timely comparison of titers and management decisions. Lab requisitions from both the mother and newborn should indicate that testing is for assessment of congenital infection.
Recommendations
1. Identify which infants should undergo a full workup and receive treatment for congenital syphilis (Figures 1 and 2)
Management decisions for the newborn must consider the details of maternal syphilis diagnosis, the adequacy of her treatment, reinfection risk, the results of clinical assessment and investigations of the infant, and the results of maternal and infant NTTs at birth. Remember that maternal NTT and TT immunoglobin G (IgG) antibodies are transferred to the fetus unless infection occurs late in the third trimester or peripartum. The following provides management guidance based on the timing of maternal diagnosis and infant clinical manifestations.
A. Syphilis diagnosed late in the third trimester, intrapartum, or immediate postpartum period
This scenario is high risk for CS. For women who present in labour who have had limited or no prenatal care and whose syphilis status is unknown, urgent syphilis testing should be sent as part of their assessment [11]. Refer to the Canadian Paediatric Society statement “Reducing perinatal infection risk in newborns of mothers who received inadequate prenatal care” to guide required testing for additional infectious diseases. When the mother’s syphilis serology is reactive, the infant should be considered at high risk for CS and receive a full assessment (see below) and course of therapy for CS regardless of the infant’s investigation results. Infants of mothers who were diagnosed later in pregnancy but before delivery, who did not complete the recommended treatment more than 4 weeks before delivery and thus did not have a documented fourfold or greater fall in NTT, should also be considered at high risk for CS and managed similarly. Clinicians should be aware that a negative syphilis test at delivery may not absolutely rule out maternal infection because an infected mother may be serologically negative for as long as 90 days following acquisition, while her infant, although exposed, would test negative. If the suspicion is high (such as if new, additional sexually transmitted infections are diagnosed at delivery), a repeat of maternal titers post-delivery may be necessary to absolutely rule out very recent maternal syphilis and direct infant follow-up.
B. Syphilis diagnosed early in pregnancy
Asymptomatic maternal syphilis may be diagnosed as part of routine prenatal screening, or women may present for assessment of an illness compatible with primary or secondary syphilis and found to be pregnant. Early antenatal detection of maternal syphilis allows for prompt initiation of treatment and reduces the risk for CS. Adequacy of maternal treatment, follow-up, and response should be confirmed in all such cases. The risk of congenital infection is high if the newborn’s serum NTT is fourfold (two dilutions) higher than the mother’s (e.g., when maternal titer is 1:4, the infant titer should be ≤1:8). Infants require a full assessment and a course of therapy for possible CS when greater than fourfold titers are present or concerns exist regarding adequacy of maternal treatment, reinfection, or findings compatible with CS on physical exam.
If the mother has been adequately treated without risk of reinfection, the paired serologies appear reassuring, and the infant’s exam is normal, then the infant can be discharged with a plan for outpatient follow-up (see “Follow-up of infants whose mothers had reactive syphilis serology”, below).
C. Mother diagnosed and treated for syphilis before the current pregnancy
As with the scenarios described above, confirmation of adequacy of previous maternal treatment and response should be obtained, along with a history focused on reinfection risk. Concurrent maternal and infant serology should be ordered and interpreted as described above. Treatment of infants for CS is not indicated when the previous maternal treatment was adequate and there are no concerns for reinfection or re-exposure, the infant exam is normal, and the paired serology results are reassuring. Infants can be discharged while awaiting their NTT results in situations where the clinician has an established, trustful clinical relationship with the infant’s caregiver(s), there are no concerns about their attending for follow-up, and the maternal NTTs at delivery do not differ significantly from results obtained after treatment. If there are follow-up concerns, the preferred strategy is to keep the baby in hospital until their NTT results become available.
D. Infant has clinical manifestations or serological findings strongly suggestive of CS (regardless of maternal treatment and treatment response)
Infants born to mothers with reactive syphilis serology who are found to have abnormal physical findings and/or investigations (such as bony changes) suggestive of CS, or who have an RPR titer ≥fourfold (or two dilutions) higher than a concurrent maternal titer, regardless of adequacy of maternal antenatal treatment, should undergo a full evaluation and receive a course of therapy for CS (Figure 2). Infants born to mothers with evidence of placental infection should also undergo full workup and therapy, even when their physical examination is unremarkable.
Figure 2. Initial management of infants born to mothers with positive syphilis serology
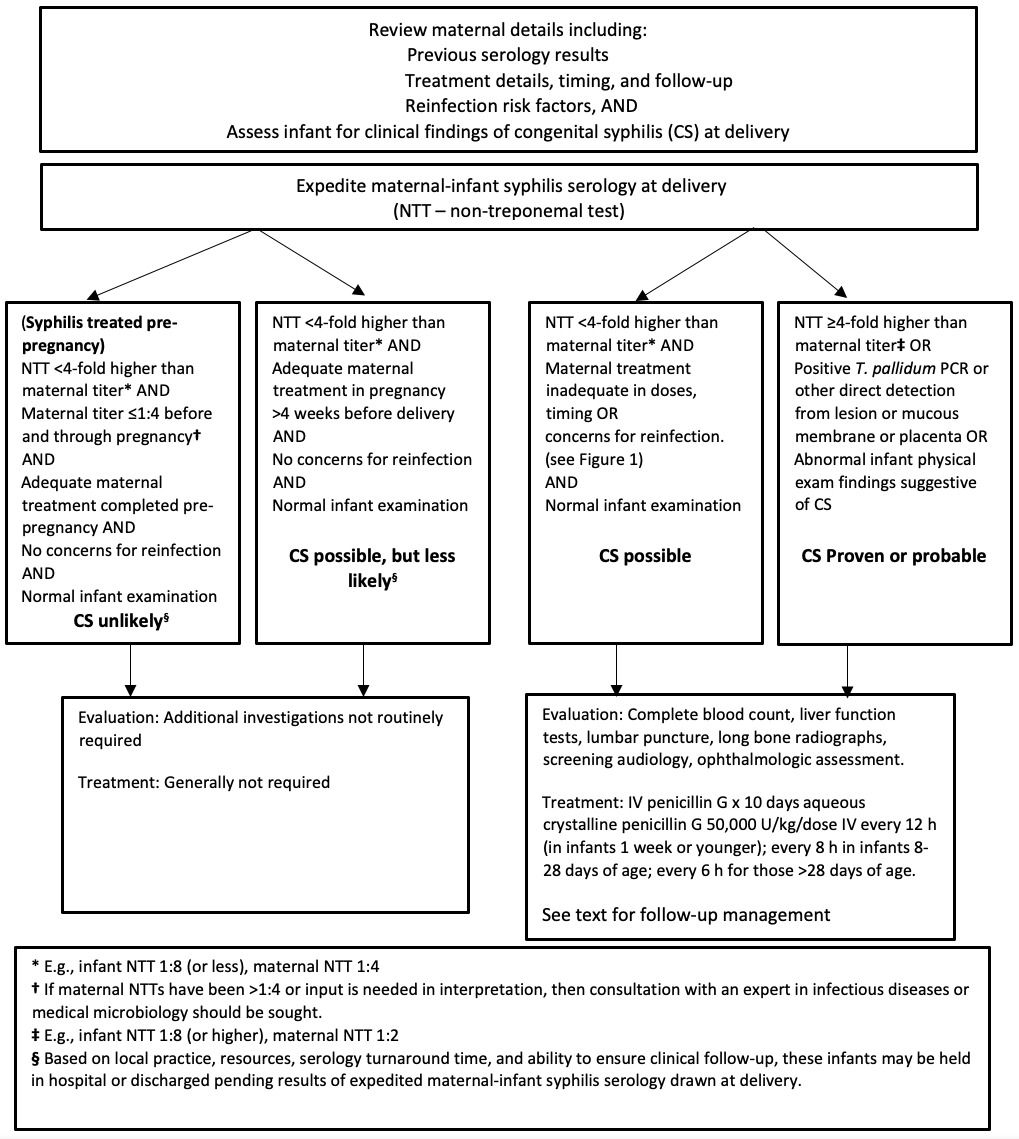
E. Infant born to mother with no antenatal care or unknown status and no availability for maternal testing
In the scenario where no maternal serology was drawn or available and the maternal serostatus is unknown, then the infant should be tested at birth[11]. Infants with reactive serology, regardless of clinical findings, should receive a full workup and be treated, with follow-up, as for any infant who completed treatment for CS. For infants who test negative initially, follow-up testing should be conducted as soon as any clinical concerns arise. If an infant remains asymptomatic, repeat testing at 3 months allows exclusion of late-trimester transmission. There is no need for follow-up serology beyond 3 months of age if the infant is asymptomatic and repeat testing remains negative.
2. Initial evaluation and treatment for CS
In higher risk scenarios (e.g., active maternal disease, inadequate treatment or NTT response to treatment, reinfection risk, clinical features of CS, or infant NTT is fourfold or greater than the maternal titer), and following a focused physical exam of the infant and paired maternal-infant syphilis serology, additional evaluations to be performed or considered are listed in Table 2.
Table 2. Recommended and additional evaluations in scenarios of high risk for congenital syphilis
| Recommended | Complete blood count (CBC) with differential and platelets |
|---|---|
| Liver function tests (e.g., ALT, AST; others as clinically indicated) | |
| CSF for cell count, differential, glucose, protein, and syphilis NTT serology | |
| Long-bone radiographs (e.g., bilateral femur and tibia/fibula) | |
| Screening audiology (auditory brain stem response) | |
| Ophthalmologic assessment* | |
| Additional investigations based on clinical indication and availability | |
| Neuroimaging† and ultrasound for organomegaly | |
| Nasopharyngeal swab and swabs of any mucosal or skin lesions for T. pallidum PCR | |
| Pathologic examination (+/− T. pallidum PCR) of the placenta for women with concerns for active infection at birth |
*Ocular syphilis can occur at any disease stage and even in otherwise asymptomatic cases but is more common in infants with neurosyphilis.
† MRI is the preferred neuroimaging modality if an infant’s CNS exam is abnormal or if neurological deficits or seizures are observed.
ALT Alanine aminotransferase, AST Aspartate aminotransferase, CSF Cerebrospinal fluid, MRI Magnetic resonance imaging, NTT Non-treponemal test, PCR Polymerase chain reaction
The treatment of choice for higher risk and confirmed cases of CS is a 10-day course of IV aqueous crystalline penicillin G, given as 50,000 units/kg/dose. Dosing frequency is age-dependent: every 12 hours during the first 7 days of life, every 8 hours for infants 8 to 28 days old, and every 6 hours for infants older than 4 weeks. Because data are scarce, the use of IV ampicillin (or other beta-lactam antibiotics) for other sepsis syndromes cannot replace the need for IV penicillin. If ampicillin is used to treat sepsis or for other reasons, and CS is highly suspected or confirmed, penicillin and ampicillin can be used concurrently while awaiting test results. Stable IV access should be secured to ensure an uninterrupted course of therapy. Some sources recommend routinely restarting the course of therapy if an interval >24 hours elapses without receiving a dose of penicillin, although the evidence supporting a restart is not clear[12]. Concerns arise if a dose is missed for >24 hours, but rather than missing further doses while awaiting IV replacement, daily IM procaine penicillin 50,000 units/kg/dose on each day that IV access is not available may be considered[12].
Infants being considered for full evaluation and treatment of CS should also be reviewed with a paediatric ID consultant. Overnight review is likely not necessary. Specialist consultation facilitates the investigations process and follow-up planning. ID consultation should also occur when concerns arise regarding: a penicillin allergy (unusual in newborns); significant adverse reactions (e.g., Jarisch-Herxheimer reaction); penicillin availability; or the interpretation of syphilis serology results. For exposed infants who are lower risk, appear well, and lack any clinical findings for CS, therapy can be held while awaiting paired serology results. If an infant is unwell, at higher risk for CS, or has clinical findings compatible with CS, initiating empiric therapy is reasonable pending further investigations.
Associations of hearing loss, ophthalmological abnormalities, and neurodevelopmental sequelae have been reported in infants with congenital neurosyphilis[13]. A lumbar puncture (LP) is recommended for confirmed or highly probable or possible cases of CS (Figure 2), because it allows confirmation of neurosyphilis. In jurisdictions where audiological, ophthalmological, and neurodevelopmental assessment may not be easily arranged, an LP will identify infants who are most in need of these assessments. For newborns in the possible CS category (Figure 2), LP is not routinely required if the infant is being treated with a 10-day course of IV penicillin G.
Some experts recommend a single dose or three weekly doses of long-acting benzathine penicillin G for infants who are not presenting with clinical or serologic evidence of CS but whose mothers appear to have received adequate antenatal treatment. However, this approach should be generally discouraged because an infant with neurosyphilis may have a falsely reassuring decline in serum titers that could delay treatment of this serious underlying infection[12]. If significant concerns exist regarding follow-up in such a scenario, then any decisions on treatment and follow-up should be undertaken in consultation with a paediatric ID specialist. Finally, when a clinician remains concerned about re-exposure and follow-up despite an asymptomatic infant and adequate serology, the preferred therapeutic option is to proceed with a full evaluation and 10 days of IV penicillin G.
3. Follow-up of infants whose mothers had reactive syphilis serology
Follow-up serology is essential for all infants born to mothers with reactive syphilis serology because maternal and infant treatment regimens do not prevent or cure every case of CS (Table 3). Infants born to mothers who seroconvert shortly after delivery should also be followed for exposure risk, with consideration of further evaluation and possible treatment based on examination and laboratory findings.
For all infants with reactive NTTs at delivery, serology should be repeated every 2 to 3 months (at least until 6 months of age), regardless of whether they received treatment or not (Figure 2). If an infant was not treated for CS at delivery (based on risk assessment criteria shown in Figures 1 and 2) then the first repeat sample should be performed around 6 weeks of age. If the infant was treated, then repeat testing could begin at 3 months of age. In uninfected infants, a reactive NTT caused by passive transfer of maternal antibody often becomes negative by 3 months of age, and should be negative by 6 months of age. Passively transferred maternal TT IgG can persist longer, but is often negative by 12 months of age, and should be negative by 18 months in all cases where the infant is not infected[14].
Table 3. Recommended serological follow-up for infants born to mothers with reactive syphilis serology
| Infection treatment status | Timing of syphilis serology* |
|---|---|
|
Not treated at delivery (due to adequate maternal treatment completed BEFORE pregnancy AND no maternal reinfection concerns post-treatment) |
Birth 3 months
6 months † 12 to 18 months |
|
Not treated at delivery (due to adequate maternal treatment completed in pregnancy >4 weeks before delivery AND no maternal reinfection concerns post-treatment) |
Birth ~6 to 8 weeks 4 months
6 months 12 to 18 months |
| Treated with 10 days IV penicillin G at delivery |
Birth 3 months
6 months 12 to 18 months |
*When the TT is nonreactive at ≥6 months of age, no additional repeat samples are warranted unless there are clinical concerns. If NTT titers increase at follow-up testing, consult ID. If NTT remains reactive >6 months, repeat testing monthly and consult with infectious diseases.
† If NTT and TT are nonreactive >3 months of age and maternal reinfection risk in late pregnancy remains low, no further testing indicated. If risk for late antenatal exposures persists, complete serial follow-up at 6 and 12 to 18 months is required.
ID Infectious diseases, NTT Non-treponemal test, TT Treponemal test
For treated infants, the NTT should follow a similar pattern of decline, although titers may take longer than 6 months to normalize if the initial NTT at delivery was high or treatment was initiated beyond the neonatal period. Consistent decline in the RPR would be reassuring in both situations. In infants with CS, TTs are expected to remain persistently positive, although some become nonreactive by 18 months[15].
For all infants, if at 6 months their NTT is nonreactive and their TT is reactive, repeat testing can be deferred until 12 months. If their NTT remains reactive without continuing to decline after 6 months or begins to increase at any point, suspect active infection. In this situation, urgent consultation with paediatric ID is warranted because repeat investigations (including LP) and treatment may be required.
For infants who have a negative NTT at delivery, follow-up testing should still occur to detect possible transmission just before delivery, as per Table 3. If their mother was adequately treated for her infection before pregnancy and there are no risk factors for relapse or reinfection, then provided the NTT at 3 months of age or later is nonreactive, further serology can be deferred until the infant is 12 to 18 months old.
When the TT is negative or nonreactive on repeat testing by 6 months of age, no additional repeat samples are warranted unless there are clinical concerns. A persistently reactive TT at or after 18 months of age confirms CS and, providing the NTT has been nonreactive, no additional repeat serology is required. These infants should be treated for CS if not already undertaken.
Following appropriate treatment of infants with CS who have abnormal long bone radiographs around delivery, repeat imaging is not routinely indicated because these signs generally resolve within the first 6 months[16]. Infants with abnormal CSF investigations at diagnosis, whose serum syphilis NTT titers decline appropriately over their first year of life, may not routinely require repeat LPs[12][17]. If their serum NTT fails to decline or if it rises at least fourfold, repeat CSF evaluation is warranted.
Collaboration among primary care clinicians, obstetrics, public health, general paediatricians, and ID specialists is critical to ensuring that all exposed children are optimally managed. In general, infants who have received a full evaluation and course of treatment for CS should have follow-up with paediatric ID or a general paediatrician in consultation with paediatric ID. For individuals receiving a new diagnosis of syphilis during pregnancy, their previous children born without confirmed negative syphilis testing near time of delivery should be re-evaluated. All infants with CS should have some form of developmental surveillance with a health care provider following their course of treatment.
Later manifestations (>2 years of age) of CS develop in a significant percentage of children when maternal syphilis and early CS are untreated. Findings relate to ongoing inflammation or scarring from earlier infection. Commonly affected body systems can include the eyes (interstitial keratitis, glaucoma, optic atrophy), bones (chronic osteochondritis or perichondritis, painless arthritis, frontal bossing, saddle nose deformity), skin (gummas, perioral fissures or scarring), and oropharynx (Mulberry molars, Hutchinson’s teeth, perforation of the hard palate)[8]. CS screening of international adoptees, immigrants, and refugees should be routine[18].
Acknowledgements
This position statement has been reviewed by the Community Paediatrics, Fetus and Newborn, and First Nations, Inuit and Métis Health Committees of the Canadian Paediatric Society. It was also reviewed by members of: the Association of Medical Microbiology and Infectious Disease (AMMI) Canada, Pediatric Committee; the Association of Ontario Midwives; the Public Health Agency of Canada’s STI Committee; and the Society of Obstetricians and Gynaecologists of Canada’s Infectious Disease Committee.
CANADIAN PAEDIATRIC SOCIETY INFECTIOUS DISEASES AND IMMUNIZATION COMMITTEE (2022)
Members: Michelle Barton MD; Ari Bitnun MD; Sergio Fanella MD; Justin Penner MD; Raphael Sharon MD (Board Representative); Laura Sauve MD (Chair); Karina A. Top MD
Liaisons: Ari Bitnun MD, Canadian Paediatric and Perinatal HIV/AIDS Research Group; Cheryl Foo MD, Immunization Monitoring Program, ACTive (IMPACT); Fahamia Koudra MD, College of Family Physicians of Canada; Yvonne Maldonado MD, Committee on Infectious Diseases, American Academy of Pediatrics; Dorothy L Moore MD, National Advisory Committee on Immunization (NACI); Marina Salvadori MD, Public Health Agency of Canada; Isabelle Viel-Thériault MD, Committee to Advise on Tropical Medicine and Travel (CATMAT)
Consultant: Noni E MacDonald MD
Authors: Sergio Fanella MD, Ari Bitnun MD, Michelle Barton MD, Laura Sauve MD
References
- Public Health Agency of Canada. Syphilis in Canada: Technical report on epidemiological trends, determinants and interventions. https://www.canada.ca/en/services/health/publications/diseasesconditions/syphilis-epidemiological-report.html#3 (Accessed November 30, 2023).
- Public Health Agency of Canada. Canadian Guidelines on Sexually Transmitted Infections. Syphilis: Key information and resources. https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/syphilis.html (Accessed November 30, 2023).
- Aho J, Lybeck C, Tetteh A, et al. Rising syphilis rates in Canada, 2011–2020. Can Comm Dis Rep 2022;48(23):52–60. doi: 10.14745/ccdr.v48i23a01.
- Manitoba Health, Seniors and Active Living. Congenital Syphilis in Manitoba. February 25, 2019. https://www.gov.mb.ca/health/publichealth/cdc/docs/hcp/2019/022519.pdf (Accessed November 30, 2023).
- Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol 2015;26 Suppl A(Suppl A):6A-12A. doi: 10.1155/2015/983425.
- Public Health Agency of Canada. Sexual Health and Sexually Transmitted Infections: Sexually transmitted and blood-borne infections: Guides for health professionals. Syphilis Guide: Treatment and follow-up. https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/syphilis/treatment-follow-up.html# (Accessed November 30, 2023).
- Woods CR. Syphilis in children: Congenital and acquired. Semin Pediatr Infect Dis 2005;16(4):245-57. doi: 10.1053/j.spid.2005.06.005.
- Heston S, Arnold S. Syphilis in children. Infect Dis Clin N Am 2018;32(1):129–44. doi: 10.1016/j.idc.2017.11.007.
- Keuning MW, Kamp GA, Schonenberg-Meinema D, Dorigo-Zetsma JW, van Zuiden JM, Pajkrt D. Congenital syphilis, the great imitator − Case report and review. Lancet Infect Dis 2020;20(7):e173-e179. doi: 10.1016/S1473-3099(20)30268-1.
- Kimball A, Bowen VB, Miele K, et al. Congenital syphilis diagnosed beyond the neonatal period in the United States: 2014-2018. Pediatrics 2021;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
- Bitnun A, Sauvé L, Fanella, S; Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Reducing perinatal infection risk in newborns of mothers who received inadequate prenatal care. https://cps.ca/en/documents/position/reducing-perinatal-infection-risk-in-newborns-of-mothers-who-received-inadequate-prenatal-care.
- Centers for Disease Control and Prevention. Sexually Transmitted Infections Treatment Guidelines, 2021. Congenital Syphilis. July 22, 2021. https://www.cdc.gov/std/treatment-guidelines/congenital-syphilis.html (Accessed November 30, 2023).
- Lim J, Yoon SJ, Shin JE, et al. Outcomes of infants born to pregnant women with syphilis: A nationwide study in Korea. BMC Pediatr 2021;21(1):47. doi: 10.1186/s12887-021-02502-9.
- Rawstron SA, Mehta S, Marcellino L, Rempel J, Chery F, Bromberg K. Congenital syphilis and fluorescent treponemal antibody test reactivity after the age of 1 year. Sex Transm Dis 2001;28(7):412-16. doi: 10.1097/00007435-200107000-00009.
- Singh AE, Guenette T, Gratrix J, et al. Seroreversion of treponemal tests in infants meeting Canadian surveillance criteria for confirmed early congenital syphilis. Pediatr Infect Dis J 2013;32(3):199-202. doi: 10.1097/INF.0b013e318273599c.
- Wilkinson RH, Heller RM. Congenital syphilis: Resurgence of an old problem. Pediatrics 1971;47(1):27-30.
- Medoro AK, Sánchez PJ. Syphilis in neonates and infants. Clin Perinatol 2021;48(2):293-309. doi: 10.1016/j.clp.2021.03.005.
- Canadian Paediatric Society. Caring for Kids New to Canada: A guide for health professionals working with immigrant and refugee children and youth. Medical Assessment of Immigrant and Refugee Children. Updated March 2023. https://kidsnewtocanada.ca/screening.
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.




