Practice point
The use of antiviral drugs for influenza: Guidance for practitioners
Posted: Oct 9, 2018 | Reaffirmed: Jan 11, 2024
Principal author(s)
Upton D. Allen; Canadian Paediatric Society, Infectious Diseases and Immunization Committee
Paediatr Child Health 2019 23(8):563. (Abstract). 
Abstract
This practice point summarizes the use of antiviral drugs to manage influenza illness in children and youth. Recommendations are based on previously published Canadian recommendations for clinicians on the use of antiviral drugs to prevent and treat influenza. Detailed information on the selective use of chemoprophylaxis can be found in the original document, which also highlights the importance of secondary bacterial infections (i.e., Streptococcus pneumoniae, methicillin-sensitive Staphylococcus aureus and methicillin-resistant S. aureus) in cases of severe influenza illness. This document serves as a reference for all clinicians while providing general principles and a user-friendly flow diagram to assist decision-making.
Keywords: Antiviral therapy; Children; Influenza; Neuraminidase inhibitors
Antiviral use in children: Past and present
Background information on seasonal influenza in children has been summarized in a full-length source document [1]. Significant issues include the comparatively high influenza rates in school-aged children [2]-[4], the atypical, non-specific nature of influenza illness in young children [5], and the high risk for adverse outcomes among children younger than 5 years of age [6]. The highest hospitalization rates occur among infants younger than 6 months of age [4]. Despite this morbidity, children of any age with mild influenza illness do not usually require antivirals. The groups at highest risk and the co-morbid conditions that predispose individuals to more severe influenza are indicated in Table 1.
Three agents are approved in Canada for use in children (Table 2). However, amantadine (for influenza A only) is not currently useful, due to resistance. The neuraminidase inhibitors (NAIs) oseltamivir (Tamiflu, Hoffman-La Roche Ltd, Canada), which is administered orally, and zanamivir (Relenza, GlaxoSmithKline Inc, United Kingdom), administered by diskhaler, are used for influenza A and B. Overall, clinical experience with NAIs is increasing [7]-[10]. Potential indications for newer NAIs that are not yet approved in Canada for use in children are becoming more apparent (Table 2) [11]-[14]. The latest addition is intravenous peramivir, which has been approved for use in the USA and Canada to treat selected cases of severe influenza illness in situations where oseltamivir and zanamavir cannot be used [11].
While specific efficacy issues have been debated in the literature on NAIs, the cumulative experience of most experts and much available data suggest that they should be used to treat all hospitalized children with influenza illness, those with underlying medical conditions and those with severe or progressive symptoms of influenza [15]. Prompt treatment may improve survival in children who are critically ill with influenza [16]. For children in outpatient settings with influenza illness, treatment with NAIs, as outlined in this document, should be considered. Overall, current evidence supports a generally favourable toxicity profile for NAIs [17].
For young children, oseltamivir is currently the most suitable agent. Oseltamivir is not approved in Canada for children younger than 1 year of age. However, during the 2009 H1N1 pandemic, oseltamivir was temporarily approved for use in this age group, based on a favourable risk-to-benefit ratio, with two studies providing valuable safety data in term [18] and preterm [19] infants. Oseltamivir use for seasonal influenza in children younger than 1 year of age should be considered on a case-by-case basis, with focus on severity of illness. Published recommendations for oseltamivir dosing in infants younger than 1 year of age vary within a reasonably narrow range [20]-[23].
While antiviral dosing for chemoprophylaxis is mentioned in the present document, such practice should be confined to specific clinical scenarios and initiated in consultation with specialists in public health or infectious diseases.
Treatment recommendations
Routes of administration and drug doses are summarized in Tables 2 and 3. A treatment algorithm is included. For explanation of levels of evidence, refer to: www.ammi.ca/Content/Guidelines/Flu%20%28published%20version%29%20FINAL.pdf
Principles for practitioners:
- Otherwise healthy individuals of any age with relatively mild, self-limited influenza are not likely to benefit from NAI therapy initiated >48 h after illness onset (Option, Grade D evidence).
Antiviral therapy should be initiated even when the interval between illness onset and administration of antiviral medication exceeds 48 h if: the illness requires hospitalization; the illness is progressive, severe or complicated, regardless of previous health status; or the individual belongs to a group at high risk for severe disease (other than young age) (Strong recommendation, Grade X evidence). This grade of evidence denotes situations where validating studies cannot be performed but there is a clear preponderance of benefit or harm.
- When the decision is made to start an antiviral, treatment should be initiated as rapidly as possible after onset of illness because the benefits of treatment are much greater with earlier initiation (within 12 h of onset) than later (at 48 h or later) (Strong recommendation, Grade B evidence).
- When results of diagnostic testing are pending, antivirals can be started but should be stopped if testing for influenza is negative.
- Parents of children for whom antiviral therapy is not recommended should be advised of the symptoms and signs of worsening illness that warrant reassessment (Recommendation, Grade D evidence).
- Routine treatment duration is for 5 days (Strong Recommendation, Grade A evidence), but may be extended in cases of severe disease (Option, Grade D evidence).
Treatment of infants, children and youth with mild or uncomplicated influenza illness (Figure 1):
Essential information is encapsulated in Figure 1, with details provided below.
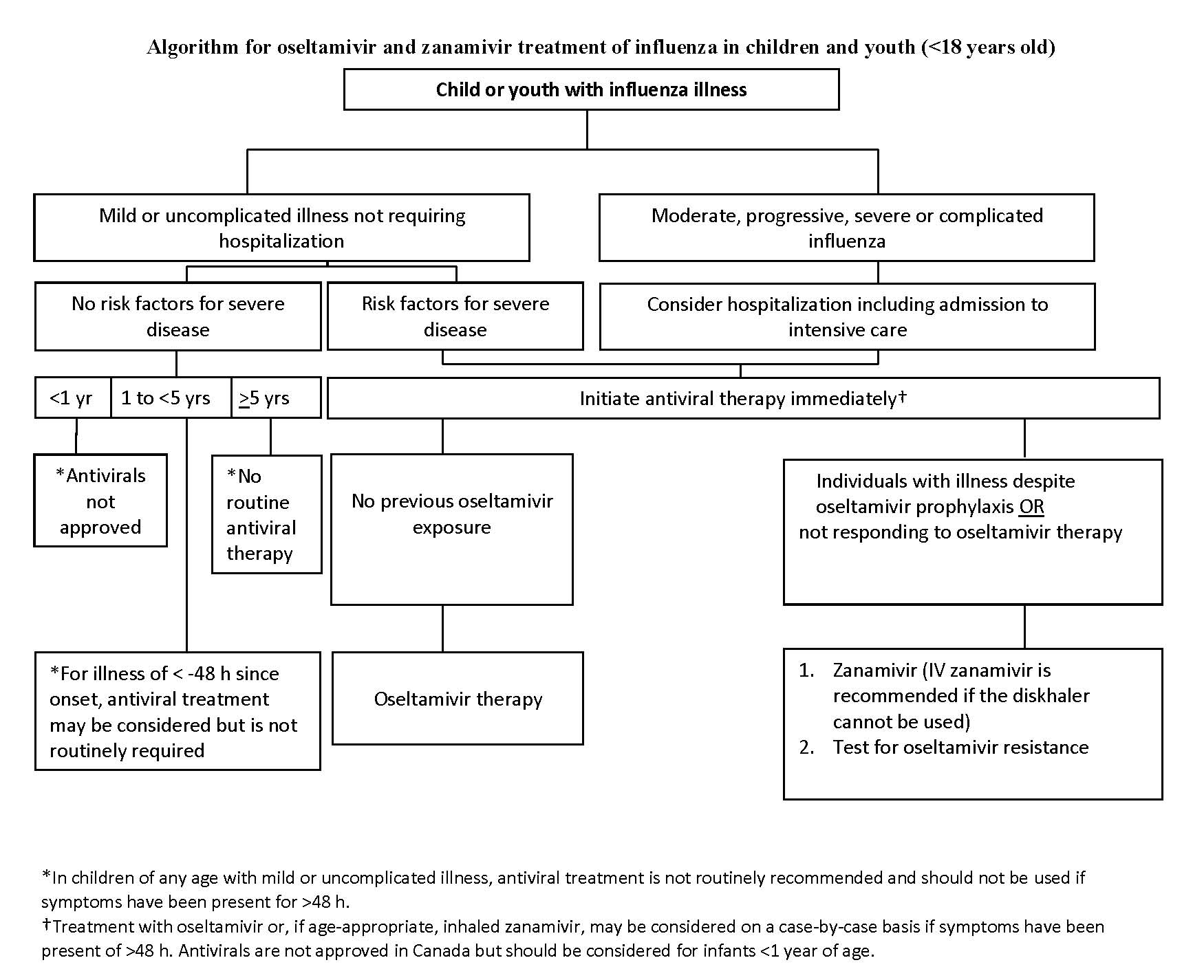
- Mild disease and no risk factors other than age:
- Younger than 1 year of age: NAIs are not approved for the routine treatment of seasonal influenza illness. Because infants <6 months old are not vaccinated for influenza, immunizing their household and other close contacts is important to protect them from disease and, thereby, to reduced the need for antiviral therapy. Influenza immunization of pregnant women should be promoted to protect infants during their first six months of life (Option, Grade D evidence).
- One to less than 5 years of age: Although children <5 years of age are classified as high risk (and those <2 years old at highest risk), individuals who are otherwise healthy, whose influenza is mild and who do not require hospitalization do not routinely require antiviral therapy. For these children, treatment is optional (Option, Grade D evidence).
- ≥5 years of age: Antiviral therapy is not routinely recommended for children and youth who are otherwise healthy, whose influenza is mild and who do not require hospitalization (Option, Grade D evidence).
- Mild disease and risk factors other than age:
- Younger than 1 year of age: NAIs are currently not approved for the routine treatment of seasonal influenza illness.
- One year of age or older: Within 48 h of illness onset, treat with oseltamivir or, when age-appropriate (>7 years), inhaled zanamivir (Recommendation, Grade B evidence).
- One year of age or older: Beyond 48 h of illness onset, treatment with oseltamivir may be considered on a case-by-case basis. When age-appropriate, inhaled zanamivir may be used instead of oseltamivir (Option, Grade D evidence).
To treat infants, children and youth with moderate, progressive, severe or complicated influenza illness, with or without risk factors:
- Evaluate the need for hospitalization and admission for intensive care (Recommendation, Grade C evidence).
- Start antivirals immediately (Strong recommendation, Grade B evidence).
- Administer oseltamivir or zanamivir in appropriate doses, even when the window between symptom onset and the starting dose of antiviral is >48 h (Recommendation, Grade C evidence).
- Consider zanamivir rather than oseltamivir for:
- Cases not responding to oseltamivir therapy (Recommendation, Grade C evidence).
- Cases with illness despite oseltamivir prophylaxis (Recommendation, Grade C evidence).
- Children <1 year old should be treated on a case-by-case basis, depending upon duration and severity of illness (Option, Grade D).
- For children who are more severely ill or who cannot tolerate oral oseltamivir, practitioners should consult an infectious diseases specialist or pharmacist for suitable alternatives.
| Table 3. Oseltamivir and zanamivir regimens | |||
|
Medication |
Treatment |
Chemoprophylaxis |
|
| Oseltamivir* | |||
| Adults | |||
|
75 mg twice daily |
75 mg once daily |
||
| Children ≥12 months | |||
|
Body weight (kg) |
Body weight (lbs) |
||
|
≤15 kg |
≤33 lbs |
30 mg twice daily |
30 mg once daily |
|
>15 kg to 23 kg |
>33 lbs to 51 lbs |
45 mg twice daily |
45 mg once daily |
|
>23 kg to 40 kg |
>51 lbs to 88 lbs |
60 mg twice daily |
60 mg once daily |
|
>40 kg |
>88 lbs |
75 mg twice daily |
75 mg once daily |
| Children 3 months to <12 months† | |||
|
3 mg/kg/dose twice daily |
3 mg/kg/dose once daily |
||
| Children <3 months‡ | |||
|
3 mg/kg/dose twice daily |
Not recommended unless situation judged critical, due to limited data on use in this age group. |
||
| Zanamivir§ | |||
| Adults | |||
|
10 mg (two 5 mg inhalations) twice daily |
10 mg (two 5 mg inhalations) once daily |
||
| Children (≥7 years for treatment and chemoprophylaxis) | |||
|
10 mg (two 5-mg inhalations) twice daily |
10 mg (two 5 mg inhalations) once daily |
||
|
Treatment regimens adapted from reference 15. Although antivirals are not authorized in Canada for the routine treatment of seasonal influenza illness in infants <1 year of age, use may be considered on a case-by-case basis. An infectious diseases specialist or clinical pharmacist should be consulted when patients with renal impairment are considered for treatment. Dose adjustments may be needed for oseltamivir, depending on creatinine clearance; no adjustment in dosing is required for inhaled zanamivir. *Oseltamivir is administered orally without reference to meals, although administration with meals may improve gastrointestinal tolerability. Oseltamivir is available in 30 mg, 45 mg and 75 mg capsules, and also as a powder for oral suspension that is reconstituted to provide a final concentration of either 6 mg/mL or 12 mg/mL. If the commercially manufactured oral suspension is not available, capsules may be opened and the contents mixed with a sweetened liquid to mask the bitter taste or a suspension can be compounded by retail pharmacies (final concentration 6 mg/ mL). When dispensing commercially manufactured oseltamivir (Tamiflu Powder for Oral Suspension (6 mg/mL or 12 mg/mL) Hoffman-La Roche Ltd, Canada), pharmacists should ensure the units of measure on the prescription instructions match the dosing device. †Weight-based dosing is preferred. Dosages recommended by the American Academy of Pediatrics [2][20] are given here to provide the clinician with an acceptable dose range: Birth to <9 months: 3 mg/kg/dose twice daily 9 to 12 months: 3.5 mg/kg/dose twice daily If body weight is not known, dosing by age to treat influenza (administer two doses per day) or prophylaxis (give one dose per day) for full-term infants <1 year of age may be necessary: 0 to 3 months = 12 mg per dose for treatment (not for prophylaxis); 3 to 5 months = 20 mg per dose; 6 to 11 months = 25 mg per dose. ‡Current weight-based dosing recommendations for oral oseltamivir are not appropriate for premature infants, who may have slower clearance because of immature renal function. Dosages recommended for full-term infants can lead to very high drug concentrations. In the USA, the Centers for Disease Control and Prevention and the American Academy of Pediatrics (Committee on Infectious Diseases, 2016) recommend dosing based on limited data from the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group: 1.0 mg/kg/dose, orally, twice daily, for premature infants born <38 weeks gestational age; 1.5 mg/kg/dose, orally, twice daily, for those 38 through 40 weeks gestational age; 3.0 mg/kg/dose, orally, twice daily, for those >40 weeks postmenstrual age. Current data are insufficient to recommend a specific dose of oseltamivir for premature infants; an infectious disease physician or clinical pharmacist should be consulted. §Zanamivir is administered by inhalation, using a proprietary ‘diskhaler’ device (Relenza, GlaxoSmithKline Inc, United Kingdom) distributed with the medication. Zanamivir is a dry powder, not an aerosol, and should not be administered using nebulizers, ventilators or other devices used to administer aerosolized medication solutions. Zanamivir is not recommended to treat individuals with chronic respiratory diseases (e.g., asthma or chronic obstructive pulmonary disease) which increase risk for bronchospasm. |
|||
Acknowledgements
This practice point was reviewed by the Acute Care, Community Paediatrics, and Drug Therapy and Hazardous Substances Committees of the Canadian Paediatric Society.
CANADIAN PAEDIATRIC SOCIETY INFECTIOUS DISEASES AND IMMUNIZATION COMMITTEE
Members: Michelle Barton-Forbes MD; Sean Bitnun MD; Natalie A. Bridger MD (past member); Shalini Desai MD (past member); Michael Forrester MD; Ruth Grimes MD (Board Representative); Nicole Le Saux MD (Chair); Karina Top MD
Liaisons: Upton D. Allen MBBS, Canadian Pediatric AIDS Research Group; Tobey Audcent MD, Committee to Advise on Tropical Medicine and Travel (CATMAT), Public Health Agency of Canada; Carrie Byington MD, Committee on Infectious Diseases, American Academy of Pediatrics; Marc Lebel MD, IMPACT (Immunization Monitoring Program, ACTIVE); Jane McDonald MD, Association of Medical Microbiology and Infectious Disease Canada; Dorothy L. Moore MD, National Advisory Committee on Immunization (NACI); Howard Njoo MD, Public Health Agency of Canada
Consultant: Noni E. MacDonald MD
Principal author: Upton D. Allen MBBS
References
- Aoki FY, Allen UD, Stiver HG, Evans GA. AMMI Canada Guideline: The use of antiviral drugs for influenza: A foundation document for practitioners. Can J Infect Dis Med Microbiol 2013;24(Suppl C):1C-15C: www.ammi.ca/Content/Guidelines/Flu%20%28published%20version%29%20FINAL.pdf (Accessed October 9, 2018).
- American Academy of Pediatrics. Influenza. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases, 31st edn. Elk Grove Village: American Academy of Pediatrics, 2018:476-90.
- Centers for Disease Control and Prevention. Influenza-associated pediatric mortality – United States: https://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html (Accessed August 14, 2018).
- Centers for Disease Control and Prevention. Clinical signs and symptoms of influenza: https://www.cdc.gov/flu/professionals/acip/clinical.htm (Accessed August 14, 2018).
- Dawood FS, Bresee J. Influenza viruses. In: Long SS, Pickering LK, Prober CG, eds. Principle and Practice of Pediatric Infectious Diseases, 5th edn. Edinburgh: Elsevier Inc, 2018:1181-1190.
- Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: A 25-year prospective study. J Infect Dis 2002;185(2):147-52.
- Kitching A, Roche A, Balasegaram S, Heathcock R, Maguire H. Oseltamivir adherence and side effects among children in three London schools affected by influenza A (H1N1)v, May 2009 – an internet-based cross-sectional survey. Euro Surveill 2009;14(30):19287.
- Barr CE, Schulman K, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr Med Res Opin 2007;23(3):523-31.
- Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics 2009;124(1):170-8.
- Gums JG, Pelletier EM, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother 2008;9(2):151-61.
- Food and Drug Administration. Emergency use authorization of peramivir IV fact sheet for health care providers: https://www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm187811.pdf (Accessed September 10, 2018).
- De Jong MD, Ison MG, Monto AS, et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis 2014:59(12):e172-85.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; Marvel Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: A double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010;51(10):1167-75.
- Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother 2010;54(6):2575-82.
- Moodley A, Bradley JS, Kimberlin DW. Antiviral treatment of childhood influenza: An update. Curr Opin Pediatr 2018;30(3):438-47.
- Louie JK, Yang S, Samuel MC, Uyeki TM, Schechter R. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics 2013;132(6):e1539-45.
- Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: A systematic review of the literature, 2009-15. J Antimicrob Chemother 2017;72(6):1556-73.
- Kimberlin DW, Shalabi M, Abzug MJ, et al. Safety of oseltamivir compared with the adamantanes in children less than 12 months of age. Pediatr Infect Dis J 2010;29(3):195-8.
- Acosta EP, Jester P, Gal P, et al. Oseltamivir dosing for influenza infection in premature neonates. J Infect Dis 2010;202(4):563-6.
- World Health Organization. WHO guidelines for pharmacological management of pandemic influenza A (H1N1) 2009 and other influenza viruses. Revised February 2010. Part I. Recommendations: http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf (Accessed September 10, 2018).
- Centers for Disease Control and Prevention. Influenza Antiviral Medications: Summary for Clinicians: www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (Accessed August 14, 2018).
- Public Health England. PHE guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza. Version 8.0, September 2017: www.gov.uk/government/uploads/system/uploads/attachment_data/file/563029/PHE_guidance_antivirals_influenza_2016_to_2017_FINAL.pdf (Accessed October 9, 2018).
- American Academy of Pediatrics. Non-HIV antiviral drugs. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book 2018 Report of the Committee on Infectious Diseases, 31st edn. Elk Grove Village: American Academy of Pediatrics, 2018:966-84.
- National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS): Canadian Immunization Guide chapter on influenza and statement on seasonal influenza vaccine for 2016-2017: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/naci-ccni/assets/pdf/flu-2016-2017-grippe-eng.pdf (Accessed August 14, 2018).
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.

