Position statement
Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures
Posted: Nov 1, 2019 | Updated: Mar 20, 2025
Principal author(s)
Evelyne D. Trottier, Marie-Joëlle Doré-Bergeron, Laurel Chauvin-Kimoff, Krista Baerg, Samina Ali, Acute Care Committee, Hospital Paediatrics Section, Community Paediatrics Section, Paediatric Emergency Medicine Section
Abstract
Common medical procedures to assess and treat patients can cause significant pain and distress. Clinicians should have a basic approach for minimizing pain and distress in children, particularly for frequently used diagnostic and therapeutic procedures. This statement focuses on infants (excluding care provided in the NICU), children, and youth who are undergoing common, minor but painful medical procedures. Simple, evidence-based strategies for managing pain and distress are reviewed, with guidance for integrating them into clinical practice as an essential part of health care. Health professionals are encouraged to use minimally invasive approaches and, when painful procedures are unavoidable, to combine simple pain and distress-minimizing strategies to improve the patient, parent, and health care provider experience. Health administrators are encouraged to create institutional policies, improve education and access to guidelines, create child- and youth-friendly environments, ensure availability of appropriate staff, equipment and pharmacological agents, and perform quality audits to ensure pain management is optimal.
Keywords: Distress; Paediatrics; Pain; Procedures; Treatment
BACKGROUND
Common medical procedures used to assess and treat patients can cause significant pain and distress, particularly in children[1][2]. Examples include intravenous (IV) cannulation, blood draws, heel lances, lumbar punctures (LPs), urethral catheterizations, wound repair, and medical imaging of fractures and dislocations. Needle-related pain is reported by children, especially among the very young, to be the worst pain they experience while in hospital[3][4]. Under-treated pain has short and long-term negative consequences for both children and their families, and can result in avoidance of medical care[4][5].
Despite many existing best practice strategies to manage pain and ample evidence for their effectiveness, suboptimal care is still consistently reported[1][2][5]-[11]. Time constraints and lack of material resources, personnel or knowledge, as well as safety concerns, are often reported as reasons for limiting the use of effective strategies[1][6][9][12][13]. Both nurses and physicians have indicated that access to synthesized, up-to-date guidelines and institutional supports would facilitate implementation of change for better pain management[6][8][13].
Addressing the pain and distress associated with common, minor medical procedures is integral to quality health care. Further, empowering the family and the child to have an active role is essential for pain management to be effective. Health professionals are encouraged to choose minimally invasive approaches and, when a painful procedure is unavoidable, to use a combination of simple strategies to improve the patient, parent, and health care provider experience[1]. Combining strategies is often more effective than using one strategy alone and can also facilitate procedural success for care providers[14]-[18]. Of course, prevention of procedural pain by avoiding unnecessary procedures is a first step to best care for children. This is the first ‘P’ of the 4P approach to pain care. This statement recommends combining the other three components—physical, psychological, and pharmacological—to minimize pain and distress. This multimodal, ‘4-P’ approach[19] should be assessed for efficacy and modified according to need, using age-appropriate pain assessment tools: www.pediadol.org/evaluation and http://www.aboutkidshealth.ca/En/ResourceCentres/Pain/PainAssessment/MeasurementofPain/Pages/Tools-For-Measuring-Pain.aspx.
This statement provides direct guidance to clinicians for managing procedural pain and distress by summarizing current evidence supporting multimodal strategies including prevention, physical, psychological, and pharmacological interventions[15]-[19]. The focus here is on minor procedures. More invasive procedures, such as circumcision[21], painful medical conditions, pain management in neonatal units[22], or IV procedural sedation and analgesia, are beyond the scope of this statement. The present statement also complements the Canadian Paediatric Society (CPS) statement on pain assessment and management published in 2022. See also the Solution for Kids In Pain website for more resources: https://kidsinpain.ca.
Physical strategies
Comfort positioning
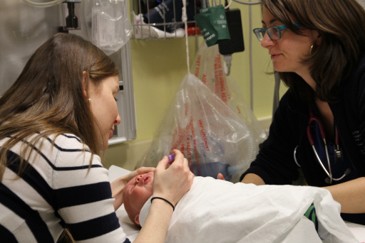
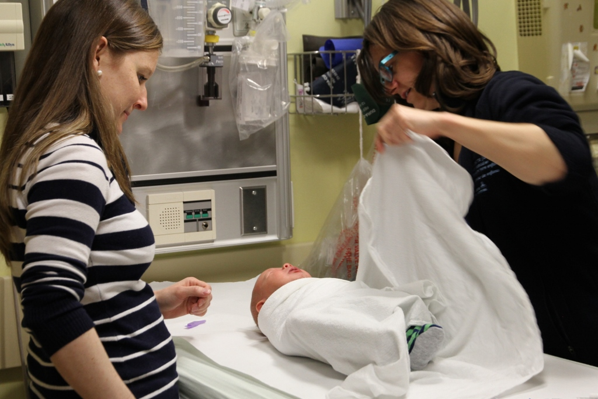
Sitting upright, rather than the traditional approach of lying on a bed while being physically restrained, has been shown to increase children’s comfort during procedures such as IV insertion or vaccination[23]-[26]. Sitting upright reduces distress by enhancing children’s sense of control. Smaller children may sit on their caregiver’s lap (Figures 1 and 2)[24]. Secure, comforting, or ‘hugging’ holds serve to assist, rather than restrain, the child[27]. Caregivers can also help support their child with distraction and soothing words while assisting with comfort positioning[28]. Family presence should always be encouraged, while taking caregiver preferences into account[1][29].
 |
 |
| Figure 1. Sitting position on caregiver’s lap; child facing the phlebotomist. |
Figure 2. Sitting position on caregiver’s lap; child facing parent. |
Infant-focused strategies
Breastfeeding can be a multimodal comfort strategy, simultaneously offering skin-to-skin contact, the comfort of sucking and rocking, and (likely) the transfer of endogenous opiates in breast milk[30]. Breastfeeding reduces procedural pain in newborns receiving heel sticks and venipunctures, as well as cry duration and pain scores during infant immunizations[30][31].
Sucrose has been studied at various dosages and concentrations[9][14][32]. For painful procedures (e.g., heel lances, venipunctures, intramuscular (IM) injections, immunization), its usefulness has been clearly shown in both preterm and term neonates[14]. For this age group, it has similar effectiveness to breastfeeding for reducing needle pain[31]. Sucrose may also reduce cry duration in infants 1 to 12 months of age[33][34], but there is insufficient evidence to support its use beyond 12 months[35]. Recommended dosing varies from 0.5 mL to 2 mL of 24% to 33% sucrose. To be most effective, part of the dose must be given 2 minutes before the procedure and the rest during the procedure (Figure 3)[9][36]. Homemade solutions can be prepared by diluting 5 g of sugar (one restaurant packet) in 10 mL of water[37]. Sucrose reduces composite pain scores by approximately 20% and is most effective when combined with other strategies[14].
Simple physical strategies such as non-nutritive sucking (i.e., pacifier use) and rocking or holding an infant can also lower pain and distress[38]-[40]. Skin-to-skin or ‘kangaroo’ care reduces pain scores in preterm and term infants undergoing painful procedures (Figure 4)[41]. Swaddling and facilitated tucking are also effective in preterm infants (Figure 5)[36]. These simple physical strategies improve the pain experience for infants at low cost and with virtually no risk. Because these strategies cannot eliminate procedural pain completely, using them in combination with pharmacotherapy, whenever possible, is recommended.
 |
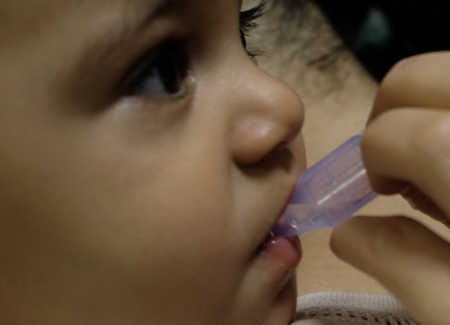 |
| Figure 3. Sucrose administration. | |
 |
 |
| Figure 4. Kangaroo care. | Figure 5. Swaddling. |
Choosing less painful approaches
When a less painful option is available, avoiding certain painful procedures, such as heel lances and IM injections, is recommended[1][5]. Since heel lancing is more painful than venipuncture, with or without sucrose, heel lances should be avoided[1][5]. When venipuncture performed by an experienced phlebotomist is compared with heel lancing, the number needed to treat to avoid repeat skin puncture is three[42]. Moreover, when both an IV insertion and blood tests are required, they should be done at the same time, whenever possible. Daily bloodwork should not be prescribed in an automatic, routine fashion. If this is required for special or critical situations, it should be restricted to a short period of time and reassessed daily. Grouping bloodwork is also important.
Psychological strategies
Preparation
There is an undeniable relationship between distress and perceived pain[1] and managing the first can directly impact the latter (see also Videos in Clinical Medicine: Managing Procedural Anxiety in Children, https://www.nejm.org/doi/full/10.1056/NEJMvcm1411127[43]). Children over the developmental age of 4 years generally benefit from simple information regarding what to expect. Explaining the steps of a procedure, receiving sensory information about what they might feel (e.g., cold, wet), seeing the medical supplies that will be used, and offering realistic choices or roles related to the procedure helps children to feel more in control[1].
Parents similarly require preparation regarding what to expect, what they can do to help with positioning and distraction, and what might be best to say during a procedure. Advise parents to avoid false or premature reassurances, such as: ‘This won’t hurt’, ‘It’s all over’, or ‘This is the last stitch’. The end of a procedure cannot always be predicted reliably, or the effectiveness of an analgesia guaranteed. Also, saying ‘I’m sorry’ can confuse a child and should be avoided in the context of performing painful procedures.
Health care providers should also be well prepared before a procedure, use proper technique, and have adequate training and knowledge to perform it. Careful, detailed explanations should be offered, to optimize informed parental consent. Level-appropriate delegation and supervision of invasive techniques in academic environments should be provided to trainees. (Instructional videos for various procedures are available at: https://www.nejm.org/multimedia/medical-videos[44].)
Distraction
Distraction strategies (e.g., blowing bubbles, reading a story, watching a program/show, an interactive game) are effective in reducing the pain and distress related to a variety of needle procedures (Figure 6)[23][45]-[48]. Distraction is the most widely studied cognitive strategy for needle-related procedural pain and distress in children ≥2 years old[45][46]. There is also good evidence that distraction can be effective during laceration repair[49][50].
A child-friendly environment, with developmentally appropriate toys, colourful wall decor, and pictures on the ceiling[1][4] helps alleviate stress and doubles as a giant ‘seek and find’ game (e.g., similar to ‘I-Spy’) that can be used in the distraction plan before and during a supine procedure (Figure 7)[51]. Web tools are available for clinicians who wish to create their own distraction kits: From SKIP’s Procedural pain management in children and youth: A toolkit for health professionals[52], see this tip-sheet from page 22[53]: https://kidsinpain.ca/wp-content/uploads/2023/04/Procedural-pain-management-in-children-and-youth.pdf and also this one in French Tout doux : Pour des soins en douceur.
For older children, the most effective distractions empower the child by asking about and attending to their preferences (either offering them an age-appropriate active distraction (e.g., an electronic game) or something more passive (e.g., a video)[54]. Engaging children in nonprocedure-related conversation also helps to shift their attention away from painful stimuli[4] and, when appropriate, humour can be used to alleviate tension. Caregivers can help with choice strategy and should be encouraged to bring items from home to distract their child (e.g., a tablet or smartphone, favourite blanket, or toy)[53].
 |
 |
| Figure 6. Distraction. | Figure 7. Overhead ‘seek and find’ posters. |
Deep breathing
Deep breathing can be used as a relaxation strategy[46] to reduce perceived pain. For example, the health care practitioner may ask the child to ‘Take a deep breath in and blow out slowly (tummy breathing)’, practice the technique with the child, and then help with prompts during the procedure.
Common tools that may help promote deep breathing and offer distraction include a pinwheel or bubble blowing (Figure 8).
Hypnosis
Hypnotic techniques, such as ‘the magic glove’[55], also reduce the pain and distress associated with needle procedures[45][46][56]. However, this technique requires specialized training and may not be suitable for busy or noisy environments (e.g., emergency departments).
Music therapy
Music therapy appears to reduce distress and pain in some children undergoing acute painful procedures[48][57][58]. Further studies are required to determine which interventions are most beneficial, which age groups are likeliest to benefit, and whether it is preferable to offer music chosen by patients or preselected by a music therapist.
 |
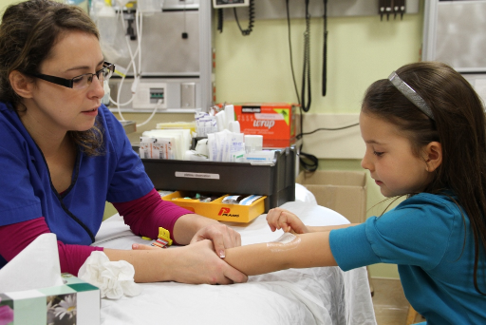 |
| Figure 8. Blowing bubbles. | Figure 9. Cream application |
Pharmacological interventions
Needle procedures
Topical local anaesthetic creams are effective for venipuncture, IV cannulation, LP, and immunization (Figure 9)[5][9][22][59]-[62] but do not seem to be effective for heel lancing[59]. Liposomal lidocaine (Maxilene) has been shown to be an effective topical anaesthetic for IV procedures[63]-[65], with shorter procedure times for IV cannulations and higher success rates on first attempts[66]. For venipuncture and cannulation, amethocaine (Ametop) is reported to be more effective than lidocaine-prilocaine (EMLA) in reducing pain, although both show effectiveness[67]. The main advantage of amethocaine and liposomal lidocaine creams is earlier onset of action compared with lidocaine-prilocaine (Table 1)[60][63]-[72]. Furthermore, reported cases of methemoglobinemia in infants with the lidocaine/prilocaine cream appear to be mainly related to the prilocaine component, which is not present in the faster acting creams[68]. Also, according to the manufacturer’s directions, occlusive dressings over liposomal lidocaine are not mandatory. When patients are stable enough to wait 30 minutes, using either amethocaine or liposomal lidocaine before needle procedures is recommended, especially when combined with other physical and psychological strategies[16][18][20][46]. Newer agents, such as Zensa (5% lidocaine) topical anesthetic cream report needing only 15 to 20 minutes application time for effect, but evidence is limited at this time.
| Table 1. Topical anaesthetics before needle procedures on intact skin | ||||
| Active ingredient (trade name) | Amide anaesthetics | Ester anaesthetic | Pentafluoropropane Tetrafluoroethane | |
| Lidocaine - prilocaine 5% (EMLA) | Liposomal lidocaine 4% (Maxilene) | Amethocaine 4% / Tetracaine (Ametop) | Vapocoolant spray (Pain Ease) | |
| Onset | 60 min (increased dermal analgesia with up to 2 h of occlusion) | 30 min | 30 min | Immediate |
| Maximal application time |
Max 4 h in children Max 1 h in ≤3 months (Max application area of 10 cm2) |
Max 2 h (Max application area 100 cm2 in <10 kg) |
Studied up to 60 min (Max dose 50 mg) | Spray 10 s or until blanching, or spray on a cloth and apply to the area (Max use twice at the same site) |
| Duration of efficacy after removal | Up to 1–2 h (efficacy increases up to 15–60 min after removal) | Longer than EMLA (efficacy increases up to 30 min after removal) | 3 h | 45–60 s |
| Advantages | No cross-sensitivity with Ametop |
Rapid action Occlusion not required No cross-sensitivity with Ametop |
Rapid action Superior to EMLA No cross-sensitivity with lidocaine |
Immediate |
| Side effects |
Vasoconstriction Methemoglobinemia (increased if <1-year-old) Hypersensitivity (rare) |
Methemoglobinemia (rare) | Hypersensitivity |
Burning sensation Frostbite |
| Contraindications | Allergy, application on mucosae or an open wound or in eyes, methemoglobinemia, G6PD. Use cautiously with heart block or severe hepatic disorder | Allergy, application on mucosae or an open wound or in eyes. Use cautiously with heart block or severe hepatic disorder | Allergy (including PABA and sulfonamides), application on mucosae or an open wound or in eyes | <4 years of age, hypersensitivity, application on mucosae or an open wound, avoid cold in children with sickle cell disease |
|
Data drawn from references[67]-[72]. |
||||
Other novel, immediately effective interventions, such as vibration/cold devices (e.g., Buzzy) or a needle-free jet injection of 1% buffered lidocaine (J-Tip) are promising options for alleviating pain during IV procedures[73]-[77] or LPs[78][79]. The J-Tip is currently unavailable in Canada. The Buzzy device is now available and has been shown in systematic review to lower pain more than no intervention. It is a promising alternative to topical anesthetic creams when timing of IV insertion does not allow for topical anesthetic effect wait time, because it is rapid, inexpensive, and easy-to-use[80]-[84]. Vapocoolant sprays (e.g., Pain Ease) may be an alternative to anesthetic creams. They have been shown to reduce pain during IV cannulation without increasing procedural difficulty[85][86]. Their main advantage is immediate effectiveness, but duration of action is limited (Table 1). They can cause mild discomfort upon application (a cold sensation)[85], which limits usefulness in younger children who cannot understand the feeling or what to expect[1].
For patients who remain distressed following the use of combination strategies described here, analgesia and anxiolysis can be achieved using agents such as nitrous oxide, in a premixed formulation of 50% nitrous oxide to 50% oxygen (Figures 10 and 11)[87][88]. Nitrous oxide is easy and safe to administer in cooperative children (usually ≥3 years old), with a rapid onset and offset of action[89]-[95]. Adding nitrous oxide to EMLA was demonstrated to provide superior analgesia in children compared with each strategy alone[87][96][97] and specifically for venous cannulation[98]. However, nitrous should be reserved when the other multimodal strategies have been shown to be insufficient, and for a short period of time with an on-demand portable system, as this is a greenhouse gas (for e.g. for specific patients, such as neuroatypical children and youth requiring a needle procedure).
Sedation for medical procedures, despite its safety when performed by trained personnel, should only be provided by clinicians with appropriate training, adequate staffing, proper equipment, access to appropriate medication and reversal agents, and monitoring. See also CPS statement on procedural sedation.
 |
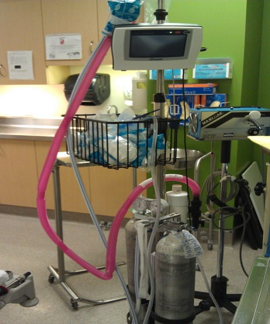 |
| Figure 10. Distraction and nitrous oxide. | Figure 11. Nitrous oxide administration. |
LP
Locally applied topical anesthetic creams (e.g., EMLA, Maxilene) and injected lidocaine have been used to successfully manage pain during LP[99][100] and to improve procedural success[101][102]. The evidence for topical anesthetic was considered uncertain in newborn in a systematic review [103]. Nevertheless, oligoanalgesia (the under-treatment of pain) for LP in infants and children is frequently reported[99][104]. For nonurgent LP, topical local anaesthetic should be administered first, followed by injected lidocaine to achieve deeper tissue anaesthetic. When urgency does not permit applying cream, about 1 mL of injected 1% lidocaine buffered with bicarbonate (1:10 ratio of biocarbonate:lidocaine), without epinephrine should still be used. Oral sucrose for infants can be added, and nitrous oxide can be a helpful adjunct for older patients (>1 year old)[105][106]. For some children, procedural sedation is required to perform the procedure.
Urine collection
Novel clean catch methods are being increasingly used as non-painful alternatives to urethral catheterization in nontoilet trained children[107]-[111]). For additional technical guidance, see: https://www.urgencehsj.ca/savoirs/prelevement-durine-clean-catch-chez-la-fille-inf/[112]. Studies of methods involving bladder stimulation using gentle tapping or cold ± paravertebral massage manoeuvres found increased procedural success rates in infants, with a reported contamination rate of approximately 15%[108].
More invasive ways to collect sterile urine include urethral catheterization and suprapubic aspiration (SPA). Simultaneous use of both topical and intraurethral 2% lidocaine gel do not seem to consistently improve urethral catheterization-associated pain management in children <2 years old[113]-[115].
Sucrose appears to have some analgesic effect in neonates but not always in older infants[116]. While lidocaine might appear to be an intuitive option to reduce pain for urethral catheterization, further studies to determine utility are needed because lidocaine does not appear to add benefit when compared with nonanaesthetic lubricants[113][117].
SPA is considered the ‘gold standard’ for obtaining sterile urine samples but also appears to be the most painful approach[118]-[120], even after a topical anaesthetic is applied[118]. Topical anaesthetic creams have been shown to reduce pain associated with SPA, but because most infants still experience pain, combining analgesia with other strategies is recommended whenever this procedure is performed[121]. Point-of-care ultrasound can also be used before SPA to confirm presence of urine and to avoid unsuccessful or ‘dry’ aspirations.
Nasogastric (NG) tube insertion
There is evidence for the efficacy of using oral sweet solutions in newborns to reduce pain before gastric tube insertion[122]. Sitting upright for NG tube placement in adults is common, along with offering water through a straw during the procedure. Both strategies may be helpful for children as well[123]. The adult medical literature also supports administering topical lidocaine before NG tube insertion[124]-[126]. Many modes of administration have been studied, including nebulized, atomized (spray), and topical lidocaine jelly, alone and in combination[124]-[126]. In children younger than 5 years old, nebulized 2% lidocaine or lidocaine/phenylephrine nasal pray did not reduce pain or distress at NG tube insertion but nebulized administration did increase distress[127][128]. However, extrapolating from the adult literature, some clinicians have chosen to introduce atomized 4% lidocaine or 1-2% of lidocaine jelly for children[28][125].
Laceration repair
Topical anaesthetics such as LET gel (lidocaine 4%/epinephrine 0.1%/tetracaine 0.5%) are recommended to reduce pain from a minor laceration before wound closure with sutures. Application should also be considered before any tissue adhesive procedure because wound cleaning, examination, and closure are facilitated with better pain management. LET is effective in 30 minutes[1][9][129] and helps achieve wound hemostasis[129]. LET is contraindicated for patients <3 months old, on mucosal surfaces and in large, deep, or contaminated wounds.
Tissue adhesives (glues) are a preferred alternative to sutures for the repair of simple, clean traumatic lacerations on tension-free surfaces[130] and reduce both procedure times and pain. Sterile adhesive strips can enhance reinforcement and mitigate the slightly increased rates of dehiscence. There is no difference in short- or long-term cosmetic outcome compared with sutures[131].
When sutures are required, prioritize absorbable sutures to avoid distress caused by suture removal[1][9][131]. Absorbable sutures are at least as good as nonabsorbable sutures for long-term cosmetic outcomes and infection rate when used in areas of low tension[1][132][133]. When LET gel is not sufficient to manage pain or a repair is urgent, local infiltration with lidocaine or a nerve block should be performed before suturing.
To reduce injection pain, bicarbonate can be added to the lidocaine in a 1:10 volume ratio. The injection solution can be warmed to body temperature and should be injected slowly, using a small gauge needle (27 G to 30 G)[1][134][135].
In addition to combined pain management strategies, some children still require short-acting anxiolysis or sedation to alleviate distress and minimize movement (e.g., intranasal (IN) midazolam[136], nitrous oxide[137][138]). Some studies have looked at the efficacy of IN sedation for laceration repair[139]-[145]. Further research is required to determine the optimal doses of IN midazolam, ketamine, and dexmedetomidine to minimize distress during different minor procedures.
Radiograph for suspected fracture or dislocation
When a fracture or a dislocation injury is suspected, analgesia combined with immobilization and icing should be provided before x-ray. Radiography is known to cause significant pain[146]. Ibuprofen appears to be superior to acetaminophen for alleviating pain associated with musculoskeletal injuries and equivalent to oral morphine[147][148]. For moderate-to-severe pain, IN fentanyl appears promising because it can be quickly administered and acts rapidly (Figure 12). IN fentanyl has been studied in the context of injuries in children[149][150]. Doses of 1 mcg/kg to 2 mcg/kg are recommended (to a maximum of 100 mcg). Monitoring after use of IN fentanyl should follow IV opioid guidelines because their systemic effects are similar[149].
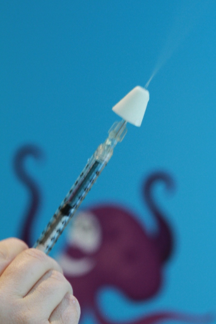 |
| Figure 12. IN fentanyl using atomizer. |
RECOMMENDATIONS FOR PHYSICIANS
Table 2 summarizes evidence-based strategies for common, minor but painful procedures. They can and should be used in combination to optimize health care outcomes and experiences.
RECOMMENDATIONS FOR HEALTH ADMINISTRATORS
Access to equitable and high‑quality pain management is a fundamental human right. Created in 2023, the Health Standards Organization (HSO’s) Pediatric Pain Management Standard (CAN/HSO 13200:2023) is the first national standard to guide pain management in the world. It guides children’s, community/regional, and rehabilitation hospitals in bettering pain care for children and youth. This national standard can be used as a template to ensure that equitable, high-quality, person-centred pain care for children and youth is provided across all settings.
The full implementation of evidence-based pain management strategies requires that multiple knowledge translation strategies be in play, supported by institutional policies and education, guideline development, child- and family-friendly environments, trained staff, unit leadership, and quality control audits[151]. Having sufficient human resources, appropriate equipment, and recommended pharmacological agents readily available is also essential. When there is institutional interest in standardizing pain management, associations such as ChildKind International (http://childkindinternational.org/) can help alongside consulting the HSO’s Pediatric Pain Management Standards.
Health care policy development should reflect commitment to pain prevention and relief at every level, with standing orders for nurses to use (for example) sucrose in all clinical settings[1] and apply topical anesthetic for selected ED patients[1][5][9][60]. Solutions for Kids In Pain has created a procedural pain management in children and youth toolkit that can be accessed here: https://kidsinpain.ca/skip-resources/.
Patients, caregivers, and staff should have ready access to educational resources on pain management strategies. Practical, evidence-based summary sheets for procedural pain can be found at kidsinpain.ca as well as TREKK.ca.
Staffing levels and training must be sufficient to support adequate pain management. A child life specialist can help caregivers and health care providers to develop coping plans using therapeutic play and family support[1][152][15]. When children do not respond to first-line strategies, specialized teams and provisions to consider a procedural sedation should be deployed. Resource allocation must permit the use of combined strategies within one or (potentially) more care centres or departments at a time, to minimize cost[36][53][131].
Finally, failure to follow standard pain management procedures should be treated as a patient safety-related event. Reporting untreated or under-treated pain as an adverse event, and viewing it from a quality lens, could inform and encourage institutional change.
Acknowledgements
This position statement has been reviewed by the Community Paediatrics and Drug Therapy and Hazardous Substances Committees of the Canadian Paediatric Society. It was also reviewed, with our thanks, by the Pediatric Section of the Canadian Association of Emergency Physicians. Use of all images is authorized by Sainte-Justine UHC (www.urgencehsj.ca) with explicit written permission to appear in this publication from parents, health professionals and Dr. G Larose.
CANADIAN PAEDIATRIC SOCIETY ACUTE CARE COMMITTEE
Members: Carolyn Beck MD, Laurel Chauvin-Kimoff MD (Chair), Kimberly Dow MD (Board Representative), Catherine Farrell MD (past member), Evelyne D. Trottier MD, Kristina Krmpotic MD, Kyle McKenzie MD
Liaisons: Kevin Chan MD, CPS Paediatric Emergency Medicine Section; Marie-Joëlle Doré-Bergeron MD, CPS Hospital Paediatrics Section
CANADIAN PAEDIATRIC SOCIETY HOSPITAL PAEDIATRICS SECTION
Executive members: Marie-Joëlle Doré-Bergeron MD (President), Jessica Foulds MD (Member at Large), Gemma Vomiero MD (Member at Large), Jennifer Walton MD (Past President), Kevin Weingarten MD (Secretary-Treasurer)
CANADIAN PAEDIATRIC SOCIETY PAEDIATRIC EMERGENCY MEDICINE SECTION
Executive members: Dominic Allain MD (Past President), Carolyn Cashin MD (Secretary-Treasurer), Kevin Chan MD (Vice President), Laurel Chauvin-Kimoff MD (President), Michelle Long MD (Member at Large), Rachel MacIntosh MD (Resident Liaison), Laura Weingarten MD (Member at Large)
CANADIAN PAEDIATRIC SOCIETY COMMUNITY PAEDIATRICS SECTION
Executive members: Anierhe E. Joan Abohweyere MD (Secretary-Treasurer), Krista Baerg MD (Past President), Karen Cozens MD (Member at Large), Carl Cummings MD (past Liaison, CPS Community Paediatrics Committee), Sarah Gander MD (Member at Large), Jane Gloor MD (Member at Large), Jennifer O’Dea MD (Member at Large), Julia Orkin MD (Liaison, CPS Community Paediatrics Committee) Liga Stare MD (President), Peter Wong MD (President-Elect)
Updated (March 2025) by: Evelyne D. Trottier MD, Marie-Joëlle Doré-Bergeron MD, Laurel Chauvin-Kimoff MD, Krista Baerg MD, Samina Ali MD
References
- Fein JA, Zempsky WT, Cravero JP; Committee on Pediatric Emergency Medicine and Section on Anesthesiology and Pain Medicine; American Academy of Pediatrics. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics 2012;130(5):e1391–405.
- Stevens BJ, Abbott LK, Yamada J, et al.; CIHR Team in Children’s Pain. Epidemiology and management of painful procedures in children in Canadian hospitals. CMAJ 2011;183(7):E403–10.
- Friedrichsdorf SJ, Postier A, Eull D, et al. Pain outcomes in a US children’s hospital: A prospective cross-sectional survey. Hosp Pediatr 2015;5(1):18–26.
- Young KD. Pediatric procedural pain. Ann Emerg Med 2005;45(2):160–71.
- Ali S, McGrath T, Drendel AL. An evidence-based approach to minimizing acute procedural pain in the emergency department and beyond. Pediatr Emerg Care 2016;32(1):36–42; quiz 43–4.
- Ali S, Chambers A, Johnson DW, et al. Reported practice variation in pediatric pain management: A survey of Canadian pediatric emergency physicians. CJEM 2014;16(5):352–60.
- Trottier ED, Ali S, Le May S, Gravel J. Treating and reducing anxiety and pain in the paediatric emergency department: The TRAPPED survey. Paediatr Child Health 2015;20(5):239–44.
- Ali S, Chambers AL, Johnson DW, et al. Paediatric pain management practice and policies across Alberta emergency departments. Paediatr Child Health 2014;19(4):190–4.
- Bailey B, Trottier ED. Managing pediatric pain in the emergency department. Paediatr Drugs 2016;18(4):287–301.
- Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in children in the emergency department. Lancet 2016;387(10013):83–92.
- Senger A, Bryce R, McMahon C, Baerg K. Cross-sectional study of pediatric pain prevalence, assessment, and treatment at a Canadian tertiary hospital. Can J Pain 2021;5(1):172-82. doi: 10.1080/24740527.2021.1961081
- Stevens BJ, Yamada J, Estabrooks CA, et al.; CIHR Team in Children’s Pain. Pain in hospitalized children: Effect of a multidimensional knowledge translation strategy on pain process and clinical outcomes. Pain 2014;155(1):60–8.
- Thomas D, Kircher J, Plint AC, et al. Pediatric pain management in the emergency department: The triage nurses’ perspective. J Emerg Nurs 2015;41(5):407–13.
- Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2016;7:CD001069.
- Leahy S, Kennedy RM, Hesselgrave J, Gurwitch K, Barkey M, Millar TF. On the front lines: Lessons learned in implementing multidisciplinary peripheral venous access pain-management programs in pediatric hospitals. Pediatrics 2008;122 (Suppl 3):S161–70.
- IWKHealthcentre IHC. It Doesn’t Have to Hurt. https://www.youtube.com/watch?v=KgBwVSYqfps (Accessed October 18, 2018).
- Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: An evidence-based clinical practice guideline (summary). CMAJ 2010;182(18):1989–95.
- Friedrichsdorf SJ; Australian Pain Society. Reducing and Eliminating Procedural Pain Related to Needles, 2014: The Four Essential (non-negotiable) Components. https://blog.apsoc.org.au/2014/08/21/reducing-and-eliminating-procedural-pain-related-to-needles-the-four-essential-non-negotiable-components/ (Accessed October 18, 2018).
- Health Standards Organization. CAN/HSO 13200:2023 – Pediatric Pain Management. https://healthstandards.org/standard/pediatric-pain-management-can-hso-13200-2023-e/ (Accessed March 31, 2025).
- American Academy of Pediatrics; Committee on Psychosocial Aspects of Child and Family Health; Task Force on Pain in Infants, Children and Adolescents. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics 2001;108(3):793–7.
- Sorokan ST, Finlay JC, Jefferies AL; Canadian Paediatric Society, Fetus and Newborn Committee, Infectious Diseases and Immunization Committee. Newborn male circumcision. Paediatr Child Health 2015;20(6):311–20.
- Batton DG, Barrington KJ, Wallman C; American Academy of Pediatrics, Committee on Fetus and Newborn, Section on Surgery; Canadian Paediatric Society, Fetus and Newborn Committee. Prevention and management of pain in the neonate: An update. Pediatrics 2006;118(5):2231–41.
- Wente SJ. Nonpharmacologic pediatric pain management in emergency departments: A systematic review of the literature. J Emerg Nurs 2013;39(2):140–50.
- Sparks LA, Setlik J, Luhman J. Parental holding and positioning to decrease IV distress in young children: A randomized controlled trial. J Pediatr Nurs 2007;22(6):440–7.
- Lacey CM, Finkelstein M, Thygeson MV. The impact of positioning on fear during immunizations: Supine versus sitting up. J Pediatr Nurs 2008;23(3):195–200.
- Taddio A, Shah V, McMurtry CM, et al.; HELPinKids&Adults Team. Procedural and physical interventions for vaccine injections: Systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain 2015;31 (10 Suppl):S20–37.
- Children’s Mercy Kansas City Child Life: Comfort Positions. 2017. https://www.childrensmercy.org/Patients_and_Families/Support_and_Services/Child_Life/Comfort_Positions/ (Accessed October 18, 2018).
- TREKK (Translating Emergency Knowledge for Kids). Bottom Line Recommendations: Procedural Pain. 2016. https://trekk.ca/system/assets/assets/attachments/164/original/procedural-pain-blr-final-reformatted-aug-2017.pdf?1505225147 (Accessed October 31, 2019).
- Ali S, Weingarten LE, Kircher J, et al. A survey of caregiver perspectives on children’s pain management in the emergency department. CJEM 2016;18(2):98–105.
- Harrison D, Reszel J, Bueno M, et al. Breastfeeding for procedural pain in infants beyond the neonatal period. Cochrane Database Syst Rev 2016;10:CD011248.
- Shah PS, Torgalkar R, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev 2023;8(8):CD004950. doi: 10.1002/14651858.CD004950.pub4
- Taddio A, Yiu A, Smith RW, Katz J, McNair C, Shah V. Variability in clinical practice guidelines for sweetening agents in newborn infants undergoing painful procedures. Clin J Pain 2009;25(2):153–5.
- Kassab M, Foster JP, Foureur M, Fowler C. Sweet-tasting solutions for needle-related procedural pain in infants one month to one year of age. Cochrane Database Syst Rev 2012;12:CD008411.
- Gouin S, Gaucher N, Lebel D, Desjardins MP. A randomized double-blind trial comparing the effect on pain of an oral sucrose solution vs. placebo in children 1 to 3 months old undergoing simple venipuncture. J Emerg Med 2018;54(1):33–9.
- Harrison D, Yamada J, Adams-Webber T, Ohlsson A, Beyene J, Stevens B. Sweet tasting solutions for reduction of needle-related procedural pain in children aged one to 16 years. Cochrane Database Syst Rev 2015;5:CD008408.
- Urgence CHU Sainte-Justine. Sucrose 0-6 mois. Révisé Janvier 2020. https://www.urgencehsj.ca/protocoles/sucrose-0-12-mois/ (Accessed March 31, 2025).
- Immunize Canada. Immunization Pain Management (Clinician Focus). 2015 https://immunize.ca/immunization-pain-management-clinician (Accessed October 18, 2018).
- Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2015;12:CD006275.
- Pillai Riddell RR, Bucsea O, Shiff I, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2023;6(6):CD006275. doi: 10.1002/14651858.CD006275.pub4
- Harrison D, Bueno M. Translating evidence: Pain treatment in newborns, infants, and toddlers during needle-related procedures. Pain Rep. 2023 Feb 16;8(2):e1064. doi: 10.1097/PR9.0000000000001064
- Johnston C, Campbell-Yeo M, Disher T, et al. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev 2017;2:CD008435.
- Shah VS, Ohlsson A. Venepuncture versus heel lance for blood sampling in term neonates. Cochrane Database Syst Rev 2011;10:CD001452.
- Krauss BS, Krauss BA, Green SM. Videos in clinical medicine. Managing procedural anxiety in children. N Engl J Med 2016;374(16):e19. https://www.nejm.org/doi/full/10.1056/NEJMvcm1411127 (Accessed October 18, 2018).
- New England Journal of Medicine. Browse Figures and Multimedia. https://www.nejm.org/multimedia/medical-videos (Accessed October 18, 2018).
- Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2013;10:CD005179.
- Birnie KA, Noel M, Chambers CT, Uman LS, Parker JA. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2018;10:CD005179.
- Chambers CT, Taddio A, Uman LS, McMurtry CM; HELPinKIDS Team. Psychological interventions for reducing pain and distress during routine childhood immunizations: A systematic review. Clin Ther 2009;31 (Suppl 2):S77–S103.
- Klassen JA, Liang Y, Tjosvold L, Klassen TP, Hartling L. Music for pain and anxiety in children undergoing medical procedures: A systematic review of randomized controlled trials. Ambul Pediatr 2008;8(2):117–28.
- Sinha M, Christopher NC, Fenn R, Reeves L. Evaluation of nonpharmacologic methods of pain and anxiety management for laceration repair in the pediatric emergency department. Pediatrics 2006;117(4):1162–8.
- Ha YO, Kim HS. The effects of audiovisual distraction on children’s pain during laceration repair. Int J Nurs Pract 2013;19 (Suppl 3):20–7.
- Equipe analgesie a l’urgence, CHU Ste-Justine. Procedures Mineures Sans Blessure : Eau. 2016. www.urgencehsj.ca/protocoles/analgesie-procedures-mineures/ (Accessed October 18, 2018).
- Solutions for Kids in Pain (SKIP), Children’s Healthcare Canada, Public Health Agency of Canada. Procedural pain management in children and youth: A toolkit for health professionals. March 2024. https://kidsinpain.ca/wp-content/uploads/2024/04/Procedural-Pain-Management-Toolkit_v2_2024_EN.pdf (Accessed April 1, 2024).
- Canadian Association of Paediatric Health Care Centres (CAPHC). Coping Strategies for Painful Procedures. https://www.childrenshealthcarecanada.ca/en/networks-and-hubs/Pediatric-Pain/FINAL_Tip-Sheet---Coping-Strategies-for-Painful-Procedures.pdf (Accessed April 1, 2024).
- Koller D, Goldman RD. Distraction techniques for children undergoing procedures: A critical review of pediatric research. J Pediatr Nurs 2012;27(6):652–81.
- Kuttner L. Pediatric hypnosis: Pre-, peri-, and post-anesthesia. Paediatr Anaesth 2012;22(6):573–7.
- Geagea D, Tyack Z, Kimble R, et al. Clinical hypnosis for procedural pain and distress in children: A scoping review. Pain Med 2023;24(6):661-702. doi: 10.1093/pm/pnac186
- Yinger OS, Gooding LF. A systematic review of music-based interventions for procedural support. J Music Ther 2015;52(1):1–77.
- Goren K, Cen Y, Montemurri V, et al. The impact of music, play, and pet therapies in managing pain and anxiety in paediatric patients in hospital: A rapid systematic review. Paediatr Child Health 2023;28(4):218-24. doi: 10.1093/pch/pxad010
- Foster JP, Taylor C, Spence K. Topical anaesthesia for needle-related pain in newborn infants. Cochrane Database Syst Rev 2017;2:CD010331.
- Rogers TL, Ostrow CL. The use of EMLA cream to decrease venipuncture pain in children. J Pediatr Nurs 2004;19(1):33–9.
- Shah V, Taddio A, McMurtry CM, et al.; HELPinKIDS Team. Pharmacological and combined interventions to reduce vaccine injection pain in children and adults: Systematic review and meta-analysis. Clin J Pain 2015;31 (10 Suppl):S38–63.
- Le May S, Wu W, Francoeur M, et al. Topical anesthetics for needle-related pain in adults and children (TOPIC): A mini-review. Front Pain Res 2024;4:1350578. doi: 10.3389/fpain.2023.1350578
- Poonai N, Alawi K, Rieder M, Lynch T, Lim R. A comparison of amethocaine and liposomal lidocaine cream as a pain reliever before venipuncture in children: A randomized control trial. Pediatr Emerg Care 2012;28(2):104–8.
- Koh JL, Harrison D, Myers R, Dembinski R, Turner H, McGraw T. A randomized, double-blind comparison study of EMLA and ELA-max for topical anesthesia in children undergoing intravenous insertion. Paediatr Anaesth 2004;14(12):977–82.
- Eichenfield LF, Funk A, Fallon-Friedlander S, Cunningham BB. A clinical study to evaluate the efficacy of ELA-max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetics cream for pain reduction of venipuncture in children. Pediatrics 2002;109(6):1093–9.
- Taddio A, Soin HK, Schuh S, Koren G, Scolnik D. Liposomal lidocaine to improve procedural success rates and reduce procedural pain among children: A randomized controlled trial. CMAJ 2005;172(13):1691–5.
- Lander JA, Weltman BJ, So SS. EMLA and amethocaine for reduction of children’s pain associated with needle insertion. Cochrane Database Syst Rev 2006;3:CD004236.
- Friedman PM, Mafong EA, Friedman ES, Geronemus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg 2001;27(12):1019–26.
- Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol 2015;31(4):450–6.
- Yip A, Soin H, Taddio A. Review of a new topical anesthetic, liposomal lidocaine, for procedural pain in children. Can J Hosp Pharmacy 2005;58(3):148–50.
- O’Brien L, Taddio A, Lyszkiewicz DA, Koren G. A critical review of the topical local anesthetic amethocaine (Ametop) for pediatric pain. Paediatr Drugs 2005;7(1):41–54.
- Children’s Hospital of Eastern Ontario. Medical Directive no. 2051. Application of Topical Anesthetics prior to Painful Procedures. 2018. http://www.cheo.on.ca/uploads/Physican/2051-CorporateDirective-TopicalAnesthetics-2018Renewal.pdf (Accessed November 16, 2018).
- Baxter AL, Cohen LL, McElvery HL, Lawson ML, von Baeyer CL. An integration of vibration and cold relieves venipuncture pain in a pediatric emergency department. Pediatr Emerg Care 2011;27(12):1151–6.
- Lunoe MM, Drendel AL, Levas MN, et al. A randomized clinical trial of jet-injected lidocaine to reduce venipuncture pain for young children. Ann Emerg Med 2015;66(5):466–74.
- Spanos S, Booth R, Koenig H, Sikes K, Gracely E, Kim IK. Jet injection of 1% buffered lidocaine versus topical ELA-max for anesthesia before peripheral intravenous catheterization in children: A randomized controlled trial. Pediatr Emerg Care 2008;24(8):511–5.
- Auerbach M, Tunik M, Mojica M. A randomized, double-blind controlled study of jet lidocaine compared to jet placebo for pain relief in children undergoing needle insertion in the emergency department. Acad Emerg Med 2009;16(5):388–93.
- Lunoe MM, Drendel AL, Brousseau DC. The use of the needle-free jet injection system with buffered lidocaine device does not change intravenous placement success in children in the emergency department. Acad Emerg Med 2015;22(4):447–51.
- Ferayorni A, Yniguez R, Bryson M, Bulloch B. Needle-free jet injection of lidocaine for local anesthesia during lumbar puncture: A randomized controlled trial. Pediatr Emerg Care 2012;28(7):687–90.
- Caltagirone R, Raghavan VR, Adelgais K, Roosevelt GE. A randomized double blind trial of needle-free injected lidocaine versus topical anesthesia for infant lumbar puncture. Acad Emerg Med 2018;25(3):310–6.
- Ballard A, Khadra C, Fortin O, et al. Cold and vibration for children undergoing needle-related procedures: A non-inferiority randomized clinical trial. Paediatr Neonatal Pain 2024;6(4):164-73. doi: 10.1002/pne2.12125
- Haidar NA, Al Amri MH, Sendad NG, Toaimah FHS. Efficacy of Buzzy device versus EMLA cream for reducing pain during needle-related procedures in children: A randomized controlled trial. Pediatr Emerg Care 2024;40(3):180-6. doi: 10.1097/PEC.0000000000002965
- Lescop K, Joret I, Delbos P, et al. The effectiveness of the BuzzyⓇ device to reduce or prevent pain in children undergoing needle-related procedures: The results from a prospective, open-label, randomised, non-inferiority study. Int J Nurs Stud 2021;113:103803. doi: 10.1016/j.ijnurstu.2020.103803
- Potts DA, Davis KF, Elci OU, Fein JA. A vibrating cold device to reduce pain in the pediatric emergency department: A randomized clinical trial. Pediatr Emerg Care 2019;35(6):419-25. doi: 10.1097/PEC.0000000000001041
- Ballard A, Khadra C, Adler S, Trottier ED, Le May S. Efficacy of the Buzzy device for pain management during needle-related procedures: A systematic review and meta-analysis. Clin J Pain 2019;35(6):532-43. doi: 10.1097/AJP.0000000000000690
- Griffith RJ, Jordan V, Herd D, Reed PW, Dalziel SR. Vapocoolants (cold spray) for pain treatment during intravenous cannulation. Cochrane Database Syst Rev 2016;4:CD009484.
- Zhu Y, Peng X, Wang S, et al. Vapocoolant spray versus placebo spray/no treatment for reducing pain from intravenous cannulation: A meta-analysis of randomized controlled trials. Am J Emerg Med 2018;36(11):2085–92.
- Hee HI, Goy RW, Ng AS. Effective reduction of anxiety and pain during venous cannulation in children: A comparison of analgesic efficacy conferred by nitrous oxide, EMLA and combination. Paediatr Anaesth 2003;13(3):210–6.
- Pedersen RS, Bayat A, Steen NP, Jacobsson ML. Nitrous oxide provides safe and effective analgesia for minor paediatric procedures—A systematic review. Dan Med J 2013;60(6):A4627.
- Roback MG, Carlson DW, Babl FE, Kennedy RM. Update on pharmacological management of procedural sedation for children. Curr Opin Anaesthesiol 2016;29 (Suppl 1):S21–35.
- Tobias JD. Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care 2013;29(2):245–65.
- Tsze DS, Mallory MD, Cravero JP. Practice patterns and adverse events of nitrous oxide sedation and analgesia: A report from the Pediatric Sedation Research Consortium. J Pediatr 2016;169:260–5.e2.
- Trottier ED, Fabrizi A, Vincent M. La sedation procedurale en salle d’urgence : Particularites pediatriques et geriatriques. Medecin du Quebec 2017;7:article 2.
- Zier JL, Liu M. Safety of high-concentration nitrous oxide by nasal mask for pediatric procedural sedation: Experience with 7802 cases. Pediatr Emerg Care 2011;27(12):1107–12.
- Veger ML, van Iterson J, Bakx R, Ridderikhof ML. The role of nitrous oxide in minor pediatric procedures in the emergency department: A systematic review. J Pediatr Surg 2024;59(6):1154-62. doi: 10.1016/j.jpedsurg.2023.12.026
- Poonai N, Creene C, Dobrowlanski A, et al. Inhaled nitrous oxide for painful procedures in children and youth: A systematic review and meta-analysis. CJEM 2023;25(6):508-28. doi: 10.1007/s43678-023-00507-0.
- Mjahed K, Sadraoui A, Benslama A, Idali B, Benaguida M. [Combination of Emla cream and nitrous oxide for venous cannulation in children]. Ann Fr Anesth Reanim 1997;16(5):488–91.
- Taddio A, McMurtry CM, Shah V, et al.; HELPinKids&Adults. Reducing pain during vaccine injections: Clinical practice guideline. CMAJ 2015;187(13):975–82.
- Ekbom K, Jakobsson J, Marcus C. Nitrous oxide inhalation is a safe and effective way to facilitate procedures in paediatric outpatient departments. Arch Dis Child 2005;90(10):1073–6.
- Gorchynski J, McLaughlin T. The routine utilization of procedural pain management for pediatric lumbar punctures: Are we there yet? J Clin Med Res 2011;3(4):164–7.
- Breakey VR, Pirie J, Goldman RD. Pediatric and emergency medicine residents’ attitudes and practices for analgesia and sedation during lumbar puncture in pediatric patients. Pediatrics 2007;119(3):e631–6.
- Baxter AL, Fisher RG, Burke BL, Goldblatt SS, Isaacman DJ, Lawson ML. Local anesthetic and stylet styles: Factors associated with resident lumbar puncture success. Pediatrics 2006;117(3):876–81.
- Goldman RD. Analgesia for lumbar puncture in infants and children. Can Fam Physician 2019;65(3):192-4.
- Pessano S, Romantsik O, Olsson E, Hedayati E, Bruschettini M. Pharmacological interventions for the management of pain and discomfort during lumbar puncture in newborn infants. Cochrane Database Syst Rev 2023;9(9):CD015594. doi: 10.1002/14651858.CD015594.pub2
- Fein D, Avner JR, Khine H. Pattern of pain management during lumbar puncture in children. Pediatr Emerg Care 2010;26(5):357–60.
- German M, Pavo MR, Palacios A, Ordonez O. Use of fixed 50% nitrous oxide-oxygen mixture for lumbar punctures in pediatric patients. Pediatr Emerg Care 2011;27(3):244–5.
- Livingston M, Lawell M, McAllister N. Successful use of nitrous oxide during lumbar punctures: A call for nitrous oxide in pediatric oncology clinics. Pediatr Blood Cancer 2017;64(11):e26610
- Herreros ML, Tagarro A, Garcia-Pose A, Sanchez A, Canete A, Gili P. Accuracy of a new clean-catch technique for diagnosis of urinary tract infection in infants younger than 90 days of age. Paediatr Child Health 2015;20(6):e30–2.
- Labrosse M, Levy A, Autmizguine J, Gravel J. Evaluation of a new strategy for cleancatch urine in infants. Pediatrics 2016;138(3):pii.e20160573.
- Lennon R, Krishnamohan A, Fitzpatrick L, Gillett M. CATCH IT: The Effect of bladder ultrasound in decreasing the time to collect a clean-catch urine sample in the nontoilet-trained child: A randomized control trial. Pediatr Emerg Care 2024;40(2):98-102. doi: 10.1097/PEC.0000000000002937
- Weill O, Labrosse M, Levy A, Desjardins MP, Trottier ED, Gravel J. Point-of-care ultrasound before attempting clean-catch urine collection in infants: A randomized controlled trial. CJEM 2019;21(5):646-52. doi: 10.1017/cem.2019.30
- Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: Randomised controlled trial. BMJ 2017;357:j1341.
- Urgence CHU Ste Justine. Prelevement d’urine par clean catch chez la fille (MD).
- Poonai N, Li J, Langford C, et al. Intraurethral lidocaine for urethral catheterization in children: A randomized controlled trial. Pediatrics 2015;136(4):e879–86.
- Mularoni PP, Cohen LL, DeGuzman M, Mennuti-Washburn J, Greenwald M, Simon HK. A randomized clinical trial of lidocaine gel for reducing infant distress during urethral catheterization. Pediatr Emerg Care 2009;25(7):439–43.
- Vaughan M, Paton EA, Bush A, Pershad J. Does lidocaine gel alleviate the pain of bladder catheterization in young children? A randomized, controlled trial. Pediatrics 2005;116(4):917–20.
- Rogers AJ, Greenwald MH, Deguzman MA, Kelley ME, Simon HK. A randomized, controlled trial of sucrose analgesia in infants younger than 90 days of age who require bladder catheterization in the pediatric emergency department. Acad Emerg Med 2006;13(6):617–22.
- Chua ME, Firaza PNB, Ming JM, Silangcruz JMA, Braga LH, Lorenzo AJ. Lidocaine gel for urethral catheterization in children: A meta-analysis. J Pediatr 2017;190:207–214.e1.
- Kozer E, Rosenbloom E, Goldman D, Lavy G, Rosenfeld N, Goldman M. Pain in infants who are younger than 2 months during suprapubic aspiration and transurethral bladder catheterization: A randomized, controlled study. Pediatrics 2006;118(1):e51–6.
- Badiee Z, Sadeghnia A, Zarean N. Suprapubic bladder aspiration or urethral catheterization: Which is more painful in uncircumcised male newborns? Int J Prev Med 2014;5(9):1125–30.
- El-Naggar W, Yiu A, Mohamed A, et al. Comparison of pain during two methods of urine collection in preterm infants. Pediatrics 2010;125(6):1224–9.
- Nahum Y, Tenenbaum A, Isaiah W, Levy-Khademi F. Effect of eutectic mixture of local anesthetics (EMLA) for pain relief during suprapubic aspiration in young infants: A randomized, controlled trial. Clin J Pain 2007;23(9):756–9.
- Chen S, Zhang Q, Xie RH, Wen SW, Harrison D. What is the best pain management during gastric tube insertion for infants aged 0-12 months: A systematic review. J Pediatr Nurs 2017;34:78–83.
- Menezes C, Breen-Reid K. Nasogastric (NG) Tube: How to Insert Your Child’s NG Tube. 2017. https://www.aboutkidshealth.ca/Article?contentid=984&language=English (Accessed October 18, 2018).
- Lor YC, Shih PC, Chen HH, et al. The application of lidocaine to alleviate the discomfort of nasogastric tube insertion: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97(5):e9746.
- Farrington M, Bruene D, Wagner M. Pain management prior to nasogastric tube placement: Atomized lidocaine. ORL Head Neck Nurs 2015;33(1):8–16.
- Kuo YW, Yen M, Fetzer S, Lee JD. Reducing the pain of nasogastric tube intubation with nebulized and atomized lidocaine: A systematic review and meta-analysis. J Pain Symptom Manage 2010;40(4):613–20.
- Babl FE, Goldfinch C, Mandrawa C, Crellin D, O’Sullivan R, Donath S. Does nebulized lidocaine reduce the pain and distress of nasogastric tube insertion in young children? A randomized, double-blind, placebo-controlled trial. Pediatrics 2009;123(6):1548–55.
- Craig SS, Seith RW, Cheek JA, et al. Lidocaine and phenylephrine versus saline placebo nasal spray for the pain and distress of nasogastric tube insertion in young children and infants: A randomised, double-blind, controlled trial. Lancet Child Adolesc Health 2019;3(6):391-7. doi: 10.1016/S2352-4642(19)30058-6
- Harman S, Zemek R, Duncan MJ, Ying Y, Petrcich W. Efficacy of pain control with topical lidocaine-epinephrine-tetracaine during laceration repair with tissue adhesive in children: A randomized controlled trial. CMAJ 2013;185(13):E629–34.
- Farion KJ, Osmond MH, Hartling L, et al. Tissue adhesives for traumatic lacerations: A systematic review of randomized controlled trials. Acad Emerg Med 2003;10(2):110–8.
- Urgence CHU Sainte-Justine. Lacération. https://www.urgencehsj.ca/protocoles/laceration/ (Accessed April 1, 2025).
- Al-Abdullah T, Plint AC, Fergusson D. Absorbable versus nonabsorbable sutures in the management of traumatic lacerations and surgical wounds: A meta-analysis. Pediatr Emerg Care 2007;23(5):339–44.
- Karounis H, Gouin S, Eisman H, Chalut D, Pelletier H, Williams B. A randomized, controlled trial comparing long-term cosmetic outcomes of traumatic pediatric lacerations repaired with absorbable plain gut versus nonabsorbable nylon sutures. Acad Emerg Med 2004;11(7):730–5.
- Cepeda MS, Tzortzopoulou A, Thackrey M, Hudcova J, Arora Gandhi P, Schumann R. Adjusting the pH of lidocaine for reducing pain on injection. Cochrane Database Syst Rev 2010;12:CD006581.
- Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg 2012;20(2):71–3.
- Rech MA, Barbas B, Chaney W, Greenhalgh E, Turck C. When to pick the nose: Out-of-hospital and emergency department intranasal administration of medications. Ann Emerg Med 2017;70(2):203–11.
- Siu A, Tran NA, Ali S, et al. Pharmacologic procedural distress management during laceration repair in children: A systematic review. Pediatr Emerg Care 2024;40(2):88-97. doi: 10.1097/PEC.0000000000003020 93.
- Poonai N, Creene C, Dobrowlanski A, et al. Inhaled nitrous oxide for painful procedures in children and youth: A systematic review and meta-analysis. CJEM 2023;25(6):508-28. doi: 10.1007/s43678-023-00507-0
- Miller JL, Capino AC, Thomas A, Couloures K, Johnson PN. Sedation and analgesia using medications delivered via the extravascular route in children undergoing laceration repair. J Pediatr Pharmacol Ther 2018;23(2):72–83.
- Ryan PM, Kienstra AJ, Cosgrove P, Vezzetti R, Wilkinson M. Safety and effectiveness of intranasal midazolam and fentanyl used in combination in the pediatric emergency department. Am J Emerg Med 2018;pii:S0735-6757(18)30413-3 (Epub ahead of print).
- Wang JY, Speechley K, Anderson KK, et al. Intranasal midazolam for procedural distress in children in the emergency department: A systematic review and meta-analysis. CJEM 2024;26(9):658-70. doi: 10.1007/s43678-024-00731-2
- Rached-d'Astous S, Finkelstein Y, Bailey B, et al. Intranasal ketamine for procedural sedation in children: An open-label multicenter clinical trial. Am J Emerg Med 2023;67:10-16. doi: 10.1016/j.ajem.2023.01.046
- Poonai N, Sabhaney V, Ali S, et al. Optimal dose of intranasal dexmedetomidine for laceration repair in children: A phase II dose-ranging study. Ann Emerg Med 2023;82(2):179-90. doi: 10.1016/j.annemergmed.2023.01.023
- Poonai N, Spohn J, Vandermeer B, et al. Intranasal dexmedetomidine for procedural distress in children: A systematic review. Pediatrics 2020;145(1):e20191623. doi: 10.1542/peds.2019-1623
- Kumar K, Ali S, Sabhaney V, et al; Pediatric Emergency Research Canada. Anxiolysis for laceration repair in children: A survey of pediatric emergency providers in Canada. CJEM 2022;24(1):75-83. doi: 10.1007/s43678-021-00210-y
- Le May S, Ali S, Plint AC, et al.; Pediatric Emergency Research Canada (PERC). Oral analgesics utilization for children with musculoskeletal injury (OUCH Trial): An RCT. Pediatrics 2017;140(5):pii:e20170186.
- Le May S, Ali S, Khadra C, et al. Pain management of pediatric musculoskeletal injury in the emergency department: A systematic review. Pain Res Manag 2016;2016:4809394.
- Poonai N, Bhullar G, Lin K, et al. Oral administration of morphine versus ibuprofen to manage postfracture pain in children: A randomized trial. CMAJ 2014;186(18):1358–63.
- Mudd S. Intranasal fentanyl for pain management in children: A systematic review of the literature. J Pediatr Health Care 2011;25(5):316–22.
- Murphy A, O’Sullivan R, Wakai A, et al. Intranasal fentanyl for the management of acute pain in children. Cochrane Database Syst Rev 2014;10:CD009942.
- Friedrichsdorf SJ, Eull D, Weidner C, Postier A. A hospital-wide initiative to eliminate or reduce needle pain in children using lean methodology. Pain Rep 2018;3 (Suppl 1):e671.
- American Academy of Pediatrics; Committee on Hospital Care and Child Life Council. Child life services. Pediatrics 2014;133(5):e1471–8.
- Mulhall A, May AL, Alexander C. Research based nursing practice–An evaluation of an educational programme. Nurse Educ Today 2000;20(6):435–42.
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.


