Position statement
Applications of point-of-care ultrasonography in the NICU: A Canadian perspective
Posted: Jul 30, 2025
Principal author(s)
Michael Narvey MD, Nina Stein MD, Yasser Elsayed MD, PhD; Canadian Paediatric Society, Fetus and Newborn Committee
Abstract
Point-of-care ultrasonography (POCUS) is an adjunctive, portable clinical assessment tool that in the hands of trained clinicians can support differential diagnosis, clarify therapeutic options, and improve key procedures. For emergent situations in the neonatal intensive care unit, POCUS can save lives when used for real-time assessment of cardiac tamponade, pleural effusions, and pneumothorax. As interest in this modality grows, formal training fellowships in both emergency medicine and intensive care have emerged in Canada, and many North American institutions are beginning to integrate POCUS into practice. This statement discusses the current state of neonatal POCUS, including challenges to implementation, offers a pathway for educational programming, and provides guidance on use with particular focus on diagnostic and procedural applications.
Keywords: Neonatal; POCUS; Ultrasonography
While the use of point-of-care ultrasonography (POCUS) began in adult medicine, interest in its neonatal applications has grown rapidly in recent years. POCUS can be life-saving when used for rapid detection of pneumothorax, pericardial effusion, internal hemorrhage, gut injury, or cardiovascular emergencies[1]. POCUS is not a replacement for routine imaging performed and interpreted by a radiologist or cardiologist, but its specific ultrasonographic views can help identify a pathophysiological state and ensure timely intervention. POCUS allows treating clinicians to integrate ultrasonographic information quickly and adjust pre-test probabilities for a wide variety of conditions[1]-[4].
To ensure safe, effective, and consistent practice, Canadian POCUS programs need to establish standards for training and care. Formal training curriculums have been developed elsewhere to achieve these goals, the details of which (and impediments to) are discussed in a ground-breaking POCUS document from the American Academy of Pediatrics (AAP)[5].
To preserve length and integrity, certain tables and figures in this statement are linked to originals in a ‘parent’ document from the American Academy of Pediatrics: Use of Point-of-Care Ultrasonography in the NICU for Diagnostic and Procedural Purposes. In such cases, table and figure numbers in the CPS statement do not correspond to those in the AAP’s technical report.
A wide range of neonatal intensive care unit (NICU) practitioners can be trained in POCUS, including subspecialty residents, paediatric residents, other medical house staff, nurses, nurse practitioners, and respiratory therapists[1][6][7]. Increasing evidence has shown that using POCUS even adjunctively can improve infant outcomes, individualize physiology-based recommendations for care, and save lives in many clinical situations[6][7].
This statement discusses the current state of neonatal POCUS, including some challenges to implementation, sketches a roadmap for educational programming, and offers guidance on use with particular focus on diagnostic and procedural applications.
Characteristics of a POCUS exam
POCUS is best viewed as an extension of the physical exam because it allows detection of pathophysiological features in real time and is therefore distinct from a typical ultrasound performed by a radiologist. POCUS assessments by a bedside clinician are performed urgently, to delineate pathophysiology, by contrast with imaging conducted by radiology at a scheduled time for routine or elective purposes. In Canada, collaborative imaging in the NICU setting should model the relationship between paediatric cardiologists involved in implementing targeted neonatal echocardiography (TNE)[6][7]. In that model, paediatric cardiologists focus on identifying structural cardiac abnormalities while those using POCUS target abnormalities in cardiac function. Also, many of the measurements obtained differ between the two groups because the goals of assessment are different. Table 1 from the AAP’s POCUS statementdraws distinctions between a neonatal POCUS practitioner and ultrasound performed by a radiologist.
Clinical scenarios for use
POCUS helps clarify etiology in critical neonatal states and is indicated in the following examples to verify whether a current care strategy is appropriate or requires changing:
- The reasons for an infant’s not responding to routine resuscitative strategies are unclear. In such cases, a focused lung, abdominal, cranial, and cardiac POCUS may help.
- Worsening oxygenation occurs in an infant not responding to non-invasive or invasive ventilatory strategies. Here, a focused lung, cardiac, and abdominal POCUS may help.
- Initial management steps are not resolving hemodynamic instability or shock, including hypotension, metabolic or lactic acidosis, or compromised renal function. A focused abdomen, cranial, and cardiac POCUS may help.
- Acute development of anemia raises strong suspicion of hemorrhage. POCUS can identify collections of blood in the intracranial, pericardial, pleural, or hepatic subcapsular, or in the peritoneal space.
Diagnostic applications
POCUS is used to obtain information that would otherwise escape detection during clinical exam.
Lung conditions
A leading advantage of ultrasonography over chest radiography is the elimination of ionizing radiation when radiographs are performed[9][10]. A competent practitioner can often confidently bypass the confirmatory radiograph, such that POCUS essentially replaces need for a radiograph. As suggested above, POCUS and radiography can be complementary modalities. Lung POCUS is useful for detecting consolidation, atelectasis, pulmonary edema, pleural effusion, and pneumothorax. Air–fluid interfaces can point toward certain clinical diagnoses based on patterns made visible by ultrasound probe. For example:
- A-lines are present in a lung that is filled with air (horizontal reflections of the pleural line in the aerated lung).
- B-lines reflect the presence of interstitial or alveolar fluid (vertical lines stretching from the pleura away from the transducer to the end of the visible image).
Pleural irregularities and hypoechoic areas may also reflect consolidation. Pleural effusions can be detected by anechoic collections above the diaphragm.
Lung POCUS is usually performed with the patient in the supine position. While any probe can be used with image depth and grain modified to demonstrate patterns, a linear probe is often preferred for clarity in practice. Four patterns of lung findings contribute to an overall severity score, with values ranging from a low of 0 to a high of 3 for each zone. Because there are three lung zones per side, scoring can range from 0 to a maximum of 18.
Figure 1 shows typical patterns and suggests interventions for common conditions in the neonate. Figure 2 from the AAP’s POCUS statement shows the lung POCUS scores and predicts responsiveness to therapeutic interventions in infants with hypoxemia. Figure 3 from the AAP’s POCUS statement describes a lung POCUS interpretation algorithm in different neonatal pulmonary conditions[11][12].
Figure 1. Four typical patterns of lung ultrasonography
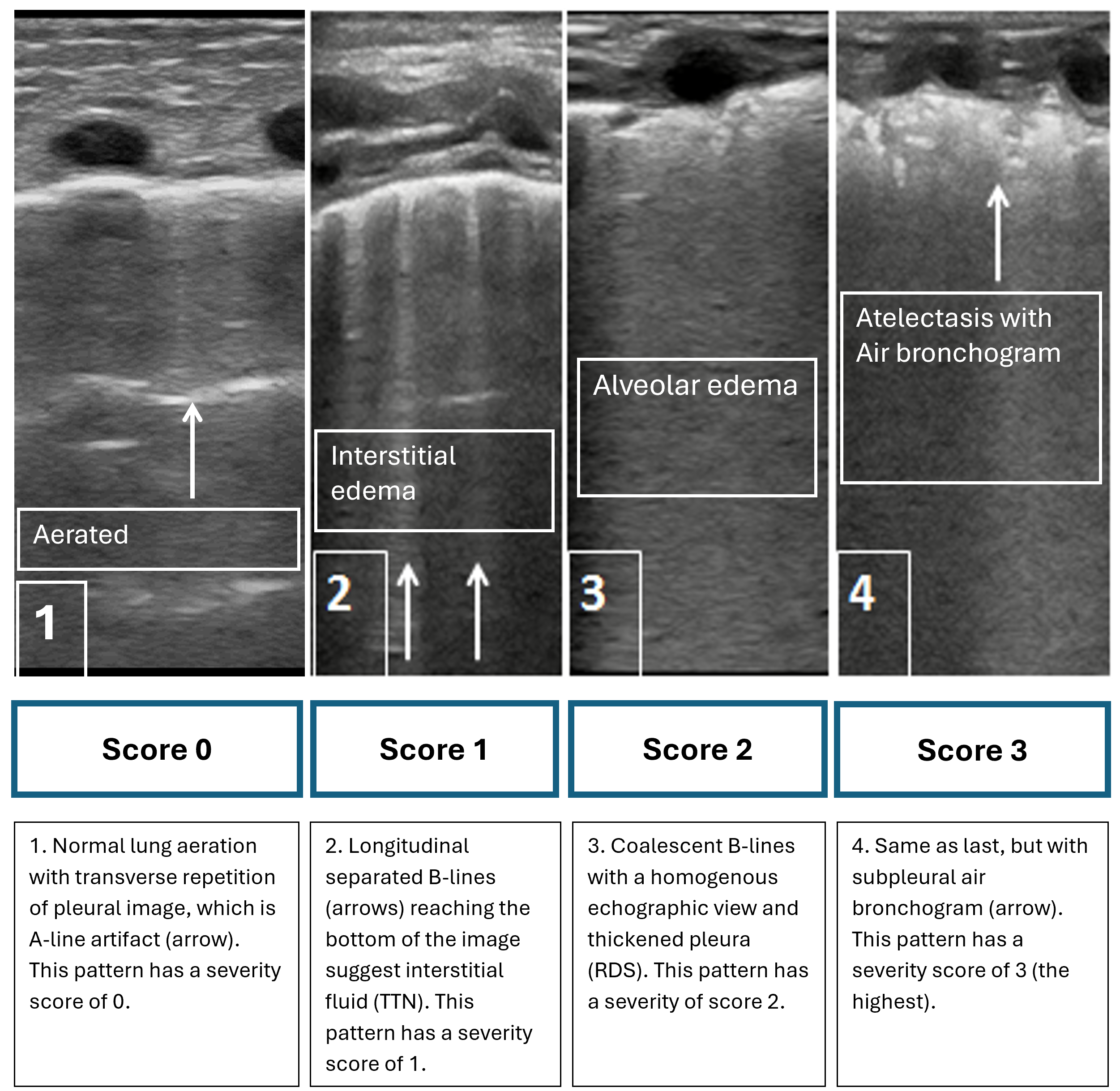
RDS Respiratory distress syndrome, TTN Transient tachypnea of the newborn
Specific considerations pertaining to the lung:[11][13][14]
- Respiratory distress syndrome has a distinctly different appearance from transient tachypnea in newborns (Table 2, scenario A, which is Table 1 in the AAP’s POCUS statement).
- When respiratory distress syndrome has been identified, POCUS can help guide the need for surfactant administration in preterm infants (Figure 4)[10][15][16].
- Pneumothorax is easily detectable, with the advantage of shorter time to diagnosis compared with traditional radiography.
Figure 4. Algorithm for lung POCUS-guided surfactant administration
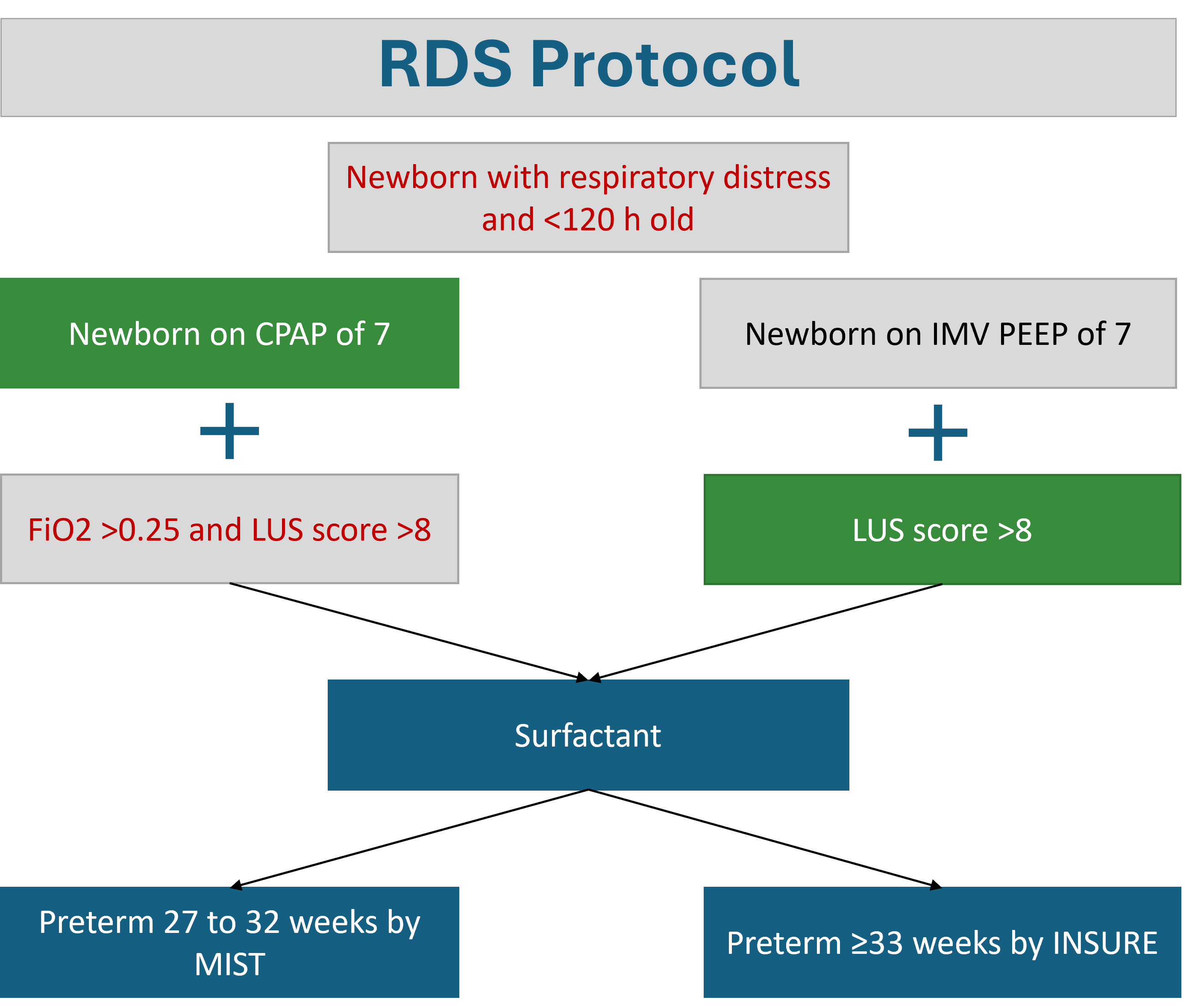
CPAP Continuous positive airway pressure, FiO2 Fraction of inspired oxygen, IMV Intermittent mechanical ventilation, INSURE Intubate surfactant extubate, LUS Lung ultrasound, MIST Minimally invasive surfactant treatment, PEEP Positive end expiratory pressure, RDS Respiratory distress syndrome
Source: Health Sciences Centre Winnipeg. With permission.
Features on ultrasonography consistent with a pneumothorax:
- No pleural sliding
- Presence of A-lines without B-lines
- The “lung point” sign separating lung tissues from the pocket of free air
- Presence of parallel horizontal lines detected by M-mode (also known as the “barcode sign”). See Table 2, scenario B, which is Table 1 in the AAP’s POCUS statement[17].
While pneumothorax may be detectable based on these signs, quantifying volume and the need for therapeutic drainage must be determined before thoracentesis or chest tube placement.
- Conditions such as pneumonia, acute pulmonary hemorrhage, or severe pulmonary edema cause consolidation of parenchymal segments in the lung. Atelectasis (Table 2, scenario D, which is Table 1 in the AAP’s POCUS statement) may yield a similar picture but with the addition of an abnormal pleural line and air bronchograms. Lung POCUS for assessment of consolidations in children of various ages has shown an 84% specificity compared with clinical assessment and chest radiography combined[18].
- Lung POCUS can identify even small amounts of pleural effusion, which typically are found in dependent areas and seen as anechoic or as spaces between the parietal and visceral pleura. Also, lung POCUS can help establish fluid characteristics. While thoracentesis is still the gold standard for differentiating transudate, exudate, or blood (i.e., hemorrhage), fluid can also look different based on how it collects (e.g., anechoic, granular, fibrinous, septated, or loculated)[1][5].
- An airway POCUS can assess for vocal cord mobility[17]. Unilateral or bilateral vocal cord paresis or paralysis can be detected by placing a linear high-frequency probe in the transverse plane at the upper level of the thyroid cartilage (submental)[19]-[20].
- Diaphragmatic motion may also be visualized by lung POCUS using either M(motion)-mode or B-mode (bidimensional imaging) in cases of suspected phrenic nerve malfunction, either in the spontaneously breathing or intubated infant.
Cardiac conditions
Cardiac POCUS has become a fundamental component for diagnosing and managing the “crashing neonate” in Canadian centres with capacity to use it. Protocols for handling such cases have been published[1].
Cardiac POCUS requires longer training and greater proficiency. Canada has two primary neonatal cardiac assessment programs. The first involves assessing suspected congenital heart disease, cardiomyopathy, or arrhythmia-related cardiac decompensation. The second is the TNE or integrated neonatal hemodynamics program, which entails a comprehensive evaluation of cardiovascular function. The latter can only be conducted by a neonatologist who has completed a fellowship in TNE and hemodynamics as per guidelines laid down by the Royal College of Physicians and Surgeons of Canada[5][20]-[22].
Abdominal conditions
POCUS provides real-time imaging of the abdominal viscera with capacity to examine peristalsis, detect minimal to significant amounts of ascites, and quantify intestinal wall thickness. POCUS can also interrogate blood flow within the intestinal wall using colour Doppler to assess for under- or overperfusion, as in inflammatory states. Radiography can only provide a single-plane snapshot of the gastrointestinal tract. It can be difficult to differentiate true pneumatosis from air along the bowel lumen on x-ray[21]-[23]. POCUS can inform degree of injury from necrotizing enterocolitis, food protein-induced enterocolitis syndrome (FPIES), or ischemic gut injury[24].
Intestinal ultrasound markers can be local or generalized to most abdominal quadrants[1][5][24]. POCUS findings should always be integrated with the clinical observations and other investigative markers, but POCUS can detect hemorrhagic ascites associated with anemia[5] as well as subcapsular hepatic or splenic hemorrhage secondary to trauma or a hemorrhagic disease of the newborn. Imaging should include the right and left hepatic lobes, spleen, and the four abdominal quadrants and midgut in two perpendicular planes (sagittal and transverse)[25]. Table 3, which is Table 2 in the AAP’s POCUS statement, describes sensitivity and specificity for diagnosis of intestinal conditions, with technical considerations. Typical findings on abdominal POCUS that indicate bowel injury are shown in Table 4[24]-[26].
| Table 4. Intestinal ultrasound markers in gut injury | |
| Pneumatosis intestinalis (PI) | |
| Strengths | Considerations |
|
|
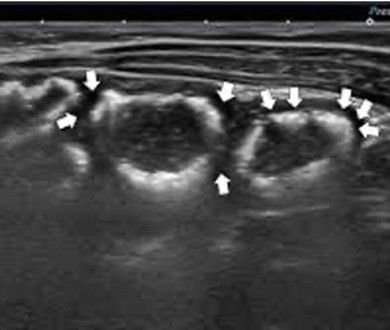 |
Arrows indicate air within the bowel wall |
| Portal venous gas (PVG) | |
| Strengths | Considerations |
|
|
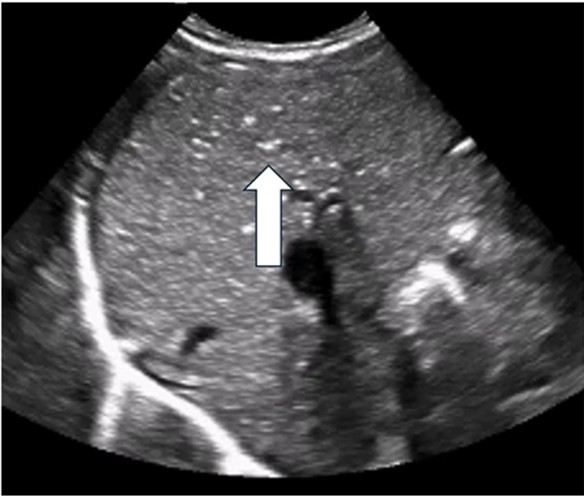 |
Arrow indicates PVG identified as echogenic bubbles in the liver |
| Bowel wall thickening/thinning | |
| Strengths | Considerations |
|
|
|
|
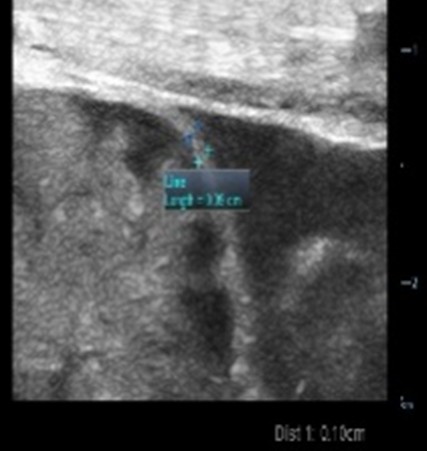 |
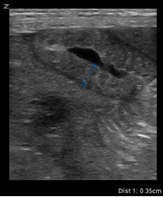
| Thickened loop of small bowel (0.35 cm) | Thinning of colonic wall |
| Peritoneal fluid/Ascites | |
| Strengths | Considerations |
|
|
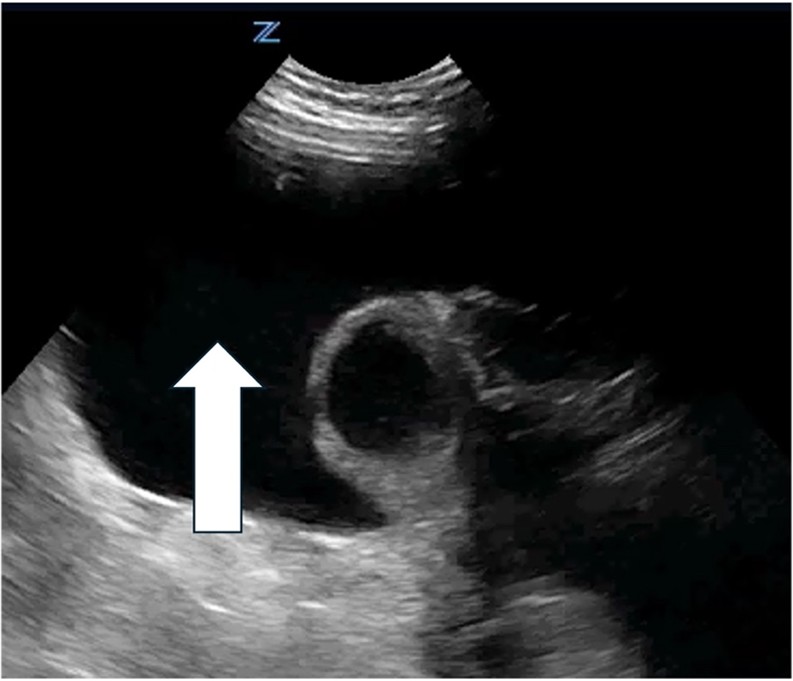 |
Arrow indicates a septated peritoneal fluid collection |
| Differentiating intestinal wall layers (known as the bowel ‘signature’) | |
| Strengths | Considerations |
|
|
 |
Magnified image demonstrates five layers of the intestinal wall, with two hyoechoic layers, the mucosa and musculosa. Then three echogenic layers being the interface between the mucosa and the lumen, submucosa, and serosa. |
| Hyperemia by colour Doppler | |
| Strengths | Considerations |
|
|
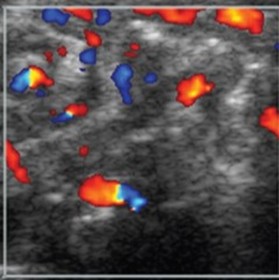  |
First: Normal intestinal image shows 3- to 9-colour speckled pattern indicating normal perfusion Second: Hyperemia with a Y pattern |
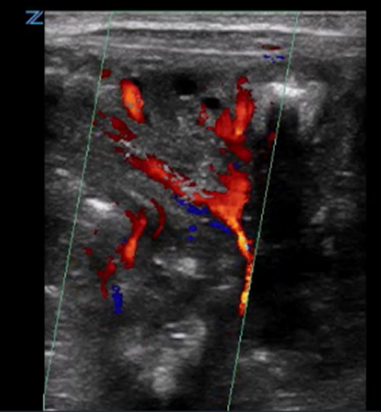
| Bowel wall ischemic gut injury by colour Doppler | |
| Strengths | Considerations |
|
|
 |
Colour speckling is not clearly identifiable |
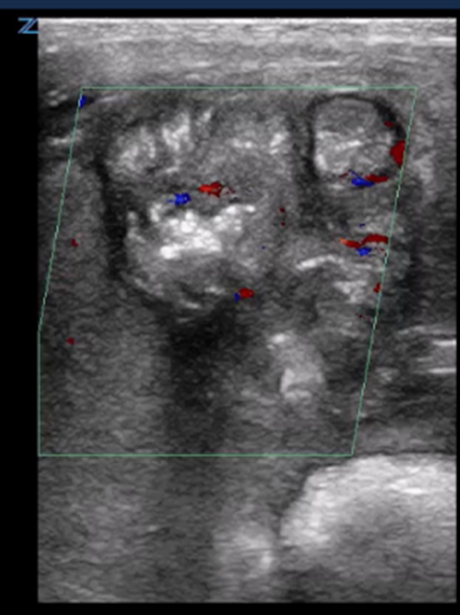
| Fixed dilated bowel loops | |
| Strengths | Considerations |
|
|
 |
Dilated small bowel loops |
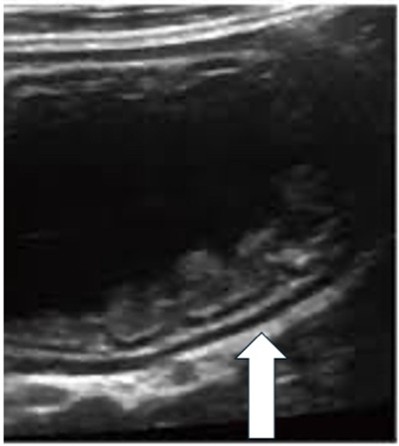
| Pneumoperitoneum | |
| Strengths | Considerations |
|
|
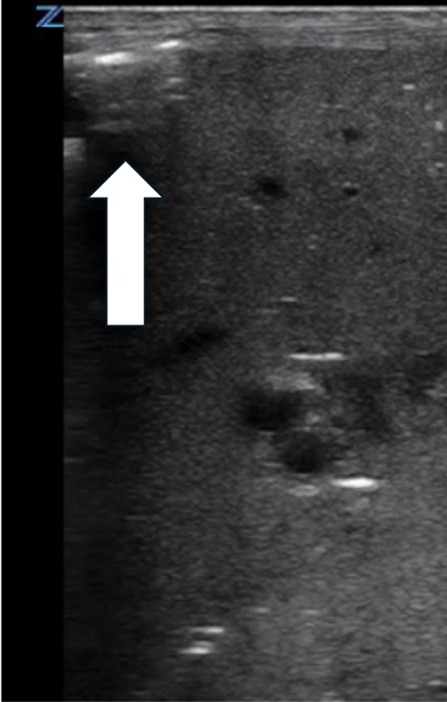 |
Small pneumoperitoneum missed on x-ray |
Additional intestinal ultrasound markers that can be detected by abdominal POCUS include absent peristalsis and increasing or decreasing blood flow, using colour Doppler[24][25]
For any POCUS application, it is essential to correlate findings with an infant’s clinical context and consult with radiology to ensure consistency.
Cranial conditions
A radiologist should perform routine cranial ultrasound to screen and assess for intraventricular hemorrhage in preterm infants. In acute cases, when intracranial hemorrhage is suspected, perform a cranial POCUS urgently, using the anterior fontanelle as the ‘acoustic window’ (Table 2, scenario H, which is Table 1 in the AAP’s POCUS statement)[4][8][27].
Urinary conditions
Assessing urine volume in the bladder can inform cases of low urine output before catheterization or suprapubic tapping for urine culture. Bladder dimensions can be measured in two or three perpendicular planes, and the volume can be estimated[2][28]. Confirm that an infant’s bladder is full using POCUS before attempting needle aspiration, both to minimize pain and the number of attempts, and to maximize urine flow[29].
Diagnostic protocols
The crashing neonate
A systematic multiorgan assessment for decompensating infants has been developed for any institution with POCUS skills. It covers four organ systems (lung, heart, abdomen, and brain[1][8]) and can be implemented in delivery rooms, NICUs, emergency departments, and other paediatric care settings. Figure 5, which is Figure 4 in the AAP’s POCUS statement, shows their stepwise approach and related interventions[1].
Interventional applications
POCUS use in neonatal units is now considered standard, safe practice in many institutions.
Guided vascular access
Using POCUS to place percutaneous central catheters (PICC lines) requires practice and skill to access the smaller veins in neonates[30]. Line tip positioning was traditionally determined using radiography, but POCUS can identify mispositioned catheters more accurately than radiography, particularly in low birth weight infants[31]-[33].
Lumbar puncture (LP)
Based on imaging obtained in both the long and short axis, spinal POCUS uses a linear transducer to identify optimal needle placement by locating the conus medullaris and determining how far from the skin to insert the needle. A real-time POCUS-guided LP technique has been described[34].
Aspiration of fluids (thoracocentesis, pericardiocentesis, and paracentesis)
Optimal visualization of fluid collection points using POCUS helps avoid injury of adjacent organs[1][5][8].
Endotracheal tube (ETT) positioning
Assessment using an ultrasound probe in the longitudinal position over the airway can help determine the distal tip of the ETT, even in premature newborns. The ETT tip can be evaluated in the suprasternal view to ensure it is positioned 1 to 2 cm above the right pulmonary artery branch[19][20].
Making POCUS available for transport teams could improve ETT-related practices and outcomes in rural and remote locations.
POCUS program implementation
POCUS training can be undertaken in centres where local expertise is lacking based on the simple, reproducible training model outlined in the European Society of Paediatric and Neonatal Intensive Care’s guidance for POCUS use in critically ill neonates and children[2]. Supporting off-site visits with experts, remote mentoring via telehealth, and holding workshops in established training centres are important first steps toward wider POCUS implementation in Canada. Institutions wishing to integrate POCUS into neonatal care must set site-specific standards for ensuring consistency of image acquisition and interpretation. Hospitals utilizing POCUS in clinical care must standardize the documentation of image findings in patient records.
Recommendations
- In the neonatal intensive care unit (NICU), consider point-of-care ultrasound (POCUS) as an extension of the newborn’s physical exam and as distinct from imaging performed by a radiologist.
- Correlate POCUS findings with an infant’s clinical context, signs, and other investigative markers, and consult with radiology to ensure consistent care.
- In Canadian NICUs, model collaborative imaging on the relationship between paediatric cardiologists involved in implementing targeted neonatal echocardiography (TNE). Cardiologists provide confirmation of structure while POCUS focuses on the function of heart and general hemodynamic status of the infant.
- Always assuming sufficient training and capacity, consider POCUS urgently when:
- Reason for an infant’s not responding to routine resuscitative strategies are unclear.
- Oxygenation is worsening despite ventilation.
- Initial management steps are not improving hemodynamic instability or shock.
- Acute anemia raises suspicion for hemorrhage.
- Always assuming sufficient training and capacity, consider POCUS to minimize infant pain and unsuccessful attempts while facilitating access for: central line insertion, endotracheal tube placement, lumbar puncture, and needle positioning to aspirate urine or other fluids.
- Use POCUS when pneumothorax, pericardial effusion, internal hemorrhage, or gut injury are suspected, and for cardiovascular emergencies.
- For lung POCUS, a linear probe is recommended for image clarity.
- When a screening ultrasound for intraventricular hemorrhage in a preterm infant raises concern, perform a cranial POCUS urgently, using the anterior fontanelle as the ‘acoustic window’.
- For abdominal conditions, use POCUS to inform degree of injury from necrotizing enterocolitis, food protein-induced enterocolitis syndrome, or ischemic gut injury.
Acknowledgements
This statement was reviewed by the Acute Care and Community Paediatrics Committees, and Neonatal-Perinatal Medicine Section Executive of the Canadian Paediatric Society, and by members of the Canadian Association of Radiologists.
CANADIAN PAEDIATRIC SOCIETY FETUS AND NEWBORN COMMITTEE (2023-2024)
Members: Michael Narvey MD (Chair), Heidi Budden MD (Board Representative), Souvik Mitra MD MSc, Eugene Ng MD, Gabriel Altit MD, Nicole Radziminski MD, Anne-Sophie Gervais MD (Resident Member)
Liaisons: William Ehman (College of Family Physicians of Canada), Chantal Nelson (Public Health Agency of Canada), Eric Eichenwald (American Academy of Pediatrics, Committee on Fetus & Newborn), Douglas Wilson (The Society of Obstetricians and Gynaecologists of Canada), Isabelle Milette (Canadian Association of Neonatal Nurses), Emer Finan MBBCH (CPS Neonatal-Perinatal Medicine Section)
Principal authors: Michael Narvey MD, Nina Stein MD, Yasser Elsayed MD, PhD
Funding
There is no funding to declare.
Potential Conflict of Interest
The authors have indicated they have no conflicts of interest.
References
- Elsayed Y, Wahab MGA, Mohamed A, et al. Point-of-care ultrasound (POCUS) protocol for systematic assessment of the crashing neonate—expert consensus statement of the international crashing neonate working group. Eur J Pediatr 2023;182(1):53-66. doi: 10.1007/s00431-022-04636-z
- Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 2020;24(1):65. doi: 10.1186/s13054-020-2787-9
- Brant JA, Orsborn J, Good R, Greenwald E, Mickley M, Toney AG. Evaluating a longitudinal point-of-care-ultrasound (POCUS) curriculum for pediatric residents. BMC Med Educ 2021;21(1):64. doi: 10.1186/s12909-021-02488-z
- Ecury-Goossen GM, Camfferman FA, Leijser LM, Govaert P, Dudink J. State of the art cranial ultrasound imaging in neonates. J Vis Exp 2015;(96):e52238. doi: 10.3791/52238
- Stewart DL, Elsayed Y, Fraga MV, Coley BD, Annam A, Milla SS; American Academy of Pediatrics, Committee on Fetus and Newborn, Section on Radiology. Clinical report: Use of point-of-care ultrasonography in the NICU for diagnostic and procedural purposes. Pediatrics 2022;150(6):e2022060052
- Elsayed Y, Sheldon J, Gigolyk S. The impact of respiratory therapist performed point-of-care lung ultrasound on the respiratory care in neonates, Manitoba experience, Canada. Am J Perinatol 2024;41(S 01):e1539-e1545. doi: 10.1055/s-0043-1768042
- Elsayed Y, Narvey M, Lashin A, Alammary D, Gigolyk S, Louis D. Point of care lung ultrasound service in neonatal intensive care: Five years of experience in Manitoba, Canada. J Perinatol 2022;42(9):1228-32. doi: 10.1038/s41372-022-01455-w
- Stewart DL, Elsayed Y, Fraga MV, Coley BD, Annam AA, Milla SS; AAP Committee on Fetus and Newborn, Section on Radiology. Technical report: Use of Point-of-Care Ultrasonography in the NICU for Diagnostic and Procedural Purposes. Pediatrics 2022;150(6):e2022060053. doi: 10.1542/peds.2022-060053
- Escourrou G, De Luca D. Lung ultrasound decreased radiation exposure in preterm infants in a neonatal intensive care unit. Acta Paediatr 2016;105(5):e237-9. doi: 10.1111/apa.13369
- Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D. Point-of-care lung ultrasound in neonatology: Classification into descriptive and functional applications. Pediatr Res 2021;90(3):524-31. doi: 10.1038/s41390-018-0114-9
- Elsayed YN, Hinton M, Graham R, Dakshinamurti S. Lung ultrasound predicts histological lung injury in a neonatal model of acute respiratory distress syndrome. Pediatr Pulmonol 2020;55(11):2913-23. doi: 10.1002/ppul.24993
- Soliman RM, Elsayed Y, Said RN, Abdulbaqi AM, Hashem RH, Aly H. Prediction of extubation readiness using lung ultrasound in preterm infants. Pediatr Pulmonol 2021;56(7):2073-80. doi: 10.1002/ppul.25383
- Corsini I, Parri N, Ficial B, Dani C. Lung ultrasound in the neonatal intensive care unit: Review of the literature and future perspectives. Pediatr Pulmonol 2020;55(7):1550-62. doi: 10.1002/ppul.24792
- Abdelmawla M, Louis D, Narvey M, Elsayed Y. A lung ultrasound severity score predicts chronic lung disease in preterm infants. Am J Perinatol 2019;36(13):1357-61. doi: 10.1055/s-0038-1676975
- De Martino L, Yousef N, Ben-Ammar R, Raimondi F, Shankar-Aguilera S, De Luca D. Lung ultrasound score predicts surfactant need in extremely preterm neonates. Pediatrics 2018;142(3):e20180463. doi: 10.1542/peds.2018-0463
- Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr 2015;169(8):e151797. doi: 10.1001/jamapediatrics.2015.1797
- Raimondi F, Rodriguez Fanjul J, Aversa S, et al. Lung ultrasound for diagnosing pneumothorax in the critically ill neonate. J Pediatr 2016;175:74-78.e1. doi: 10.1016/j.jpeds.2016.04.018
- Santos TM, Franci D, Coutinho CMG, et al. A simplified ultrasound-based edema score to assess lung injury and clinical severity in septic patients. Am J Emerg Med 2013;31(12):1656-60. doi: 10.1016/j.ajem.2013.08.053
- Chowdhry R, Dangman B, Pinheiro JMB. The concordance of ultrasound technique versus X-ray to confirm endotracheal tube position in neonates. J Perinatol 2015;35(7):481-4. doi: 10.1038/jp.2014.240
- de Kock SH, Otto SF, Joubert G. The feasibility of determining the position of an endotracheal tube in neonates by using bedside ultrasonography compared with chest radiographs. SAJCH 2015;9(1):3-5. doi: 10.7196/SAJCH.740
- Becker BA, Lahham S, Gonzales MA, et al. A prospective, multicenter evaluation of point-of-care ultrasound for small-bowel obstruction in the emergency department. Acad Emerg Med 2019;26(8):921-30. doi: 10.1111/acem.13713
- Cuna AC, Lee JC, Robinson AL, Allen NH, Foley JE, Chan SS. Bowel ultrasound for the diagnosis of necrotizing enterocolitis: A meta-analysis. Ultrasound Q 2018;34(3):113-18. doi: 10.1097/RUQ.0000000000000342
- Raghuveer TS, Lakhotia R, Bloom BT, Desilet-Dobbs DA, Zarchan AM. Abdominal ultrasound and abdominal radiograph to diagnose necrotizing enterocolitis in extremely preterm infants. Kansas J Med 2019;12(1):24-7. doi: 10.17161/kjm.v12i1.11707
- Elsayed Y, Seshia M. A new intestinal ultrasound integrated approach for the management of neonatal gut injury. Eur J Pediatr 2022;181(4):1739-49. doi: 10.1007/s00431-021-04353-z
- Elsayed Y, Louis D, Hinton M, Seshia M, Alvaro R, Dakshinamurti S. A novel integrated clinical-biochemical-radiological and sonographic classification of necrotizing enterocolitis. Am J Perinatol 2024;41(S 01):e3401-12. doi: 10.1055/s-0043-1778666
- Elsayed Y, Soylu H. Point-of-care abdominal ultrasound in pediatric and neonatal intensive care units. Eur J Pediatr 2024;183(5):2059-69. doi: 10.1007/s00431-024-05443-4
- van Wezel-Meijler G, Steggerda SJ, Leijser LM. Cranial ultrasonography in neonates: Role and limitations. Semin Perinatol 2010;34(1):28-38. doi: 10.1053/j.semperi.2009.10.002
- Miller LE, Stoller JZ, Fraga MV. Point-of-care ultrasound in the neonatal ICU. Curr Opin Pediatr 2020;32(2):216-27. doi: 10.1097/MOP.0000000000000863
- Kiernan SC, Pinckert TL, Keszler M. Ultrasound guidance of suprapubic bladder aspiration in neonates. J Pediatr 1993;123(5):789-91. doi: 10.1016/S0022-3476(05)80861-3
- Barone G, Pittiruti M, Ancora G, Vento G, Tota F, D’Andrea V. Centrally inserted central catheters in preterm neonates with weight below 1500 g by ultrasound-guided access to the brachio-cephalic vein. J Vasc Access 2021;22(3):344-52. doi: 10.1177/1129729820940174
- Fraga MV, Stoller JZ, Glau CL, et al. Seeing is believing: Ultrasound in pediatric procedural performance. Pediatrics 2019;144(5):e20191401. doi: 10.1542/peds.2019-1401
- Nguyen J. Ultrasonography for central catheter placement in the neonatal intensive care unit—A review of utility and practicality. Am J Perinatol 2016;33(6):525-30. doi: 10.1055/s-0035-1569987
- Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol 2013;33(10):791-4. doi: 10.1038/jp.2013.58
- Stoller JZ, Fraga MV. Real-time ultrasound-guided lumbar puncture in the neonatal intensive care unit. J Perinatol 2021;41(10):2495-8. doi: 10.1038/s41372-021-01152-0
Disclaimer: The recommendations in this position statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. Internet addresses are current at time of publication.